Kamagra Soft
Kamagra Soft dosages: 100 mg
Kamagra Soft packs: 10 pills, 30 pills, 60 pills, 90 pills, 120 pills, 180 pills

Kamagra soft 100 mg mastercard
A new polymer impotence high blood pressure buy kamagra soft 100 mg online, brominated isobutylene paramethylstyrene terpolymer erectile dysfunction treatment by ayurveda kamagra soft 100 mg buy cheap on-line, was just lately introduced and is said to yield very low levels of extractables, which heretofore have only been possible with polymer-coated stoppers [46]. Rubber consists of a number of materials, every of which is necessary for a particular bodily or chemical property. A rubber formulation may comprise multiple elastomer; blends of natural and chlorobutyl are frequent. More than one pigment may be used; mixtures of carbon black and titanium dioxide are used to produce gray-colored rubber components. Similarly, a number of types of other supplies may be used additionally; therefore, a rubber formulation might contain many ingredients, each of which may contribute to leachables in drug merchandise. A typical chlorobutyl pharmaceutical formulation, materials, and percentage are listed beneath in Table 25. It possesses distinctive bodily properties which might be necessary to the functions of the whole parenteral packaging system. Even earlier than that, soon after the invention of the vulcanization process by Charles Goodyear in 1839, using rubber in medical functions similar to bandages, gloves, tubing, sizzling water bottles, and syringes was described [44,45]. These natural solvents can extract larger amounts of and greater numbers of organic compounds from rubber packaging elements than aqueous solutions generally utilized for injectable or ophthalmic drugs. Products which are terminally sterilized by heat are especially vulnerable to greater leachables since the drug product is in direct contact with packaging parts at 121�C or higher for 30 or more minutes. Refrigerated (2�C�8�C) and frozen (-25�C to -10�C) products mitigate the rate of migration of packaging extractables. Transport and storage at temperatures larger than that beneficial on the label not only impacts the stability of the product but in addition may enhance leachables [49]. The evaluation of extractables and leachables from rubber is especially challenging for the next reasons: � the composition of rubber is usually proprietary. Therefore, getting details about potential extractables from suppliers is tough. Drug producers generally must perform extractables studies previous to leachables research to find a way to qualify rubber components. It is beneficial that the first step in any E&L examine is contact with the provider to get as a lot rubber composition data as attainable. Some of the monomers and oligomers are risky and may migrate from rubber stoppers into dry lyophilized drug products throughout storage [46]. Many elements affect both the number of chemical compounds and their quantities extracted from rubber right into a drug product. These elements are as follows: � Type of rubber formulation-bromobutyl rubbers are typically "cleaner" (have lower extractables) than other rubber types within the order: bromobutyls > chlorobutyls > butyls > pure. Modern rubber formulations might have solely 6�8 ingredients whereas older natural rubber formulations have 10�15 ingredients. The crosslink in thermoset polymers is a covalent bond created through the curing process. Due to the absences of a chemical curing system, thermoplastics have easier chemistries than thermosets and potentially lower ranges of leachables. Unfortunately, thermoplastics have found limited use to date as pharmaceutical packaging parts as a result of their tendency to deform during terminal sterilization. During the vulcanization or crosslinking process, reactive curing agents, accelerators, and antioxidants are chemically changed; antioxidants continue to change publish manufacturing as they react with oxygen to shield the polymer. These factors make identification, quantification, and qualification very difficult. Thermosets are polymers which were chemically crosslinked to form the ultimate structure of the material. Crosslinking and forming of the rubber into a functionally formed packaging component happen in a mould within the presence of heat and pressure. The necessity of a curing system (curing agent, Reduction of Leachables Leachables are an inevitable companion of packaging, however one can take steps to cut back them to ranges which are safe for the aim intended. Mix a portion of the extract with drug product and observe/analyze product for interactions. A typical 20 mm vial stopper has greater than twice the drug product contact surface space than a 13 mm stopper. Limit contact time between drug product and rubber part by limiting the shelf life. Pre-extract rubber elements earlier than use to scale back the amount of substances obtainable for migration into drug products. This technique was quite common for pure rubber vial stoppers and syringes when older sulfur-containing remedy techniques have been used. The introduction of synthetic halobutyl stoppers with cleaner curing methods this practice has become much less common. However, for rubber components used in contact natural solvents, corresponding to valves and o-rings in inhalation drug containers, pre-extraction of elements remains to be generally used [13]. Freezedrying, refrigerating, and freezing the drug product will cut back the rate of extraction of impurities from Parenteral Medications rubber. When undesirable drug product�packaging interactions are anticipated, avoidance of terminal sterilization in favor of aseptic processing is really helpful. Coated stoppers are commercially obtainable in two types: partially coated and totally coated. In the partially coated kind, a thin movie of inert polymer is laminated onto the surface of the rubber closure in the molding process. In the Helvoet course of, stoppers are coated on all surfaces with a fluoropolymer materials after the stopper is molded. Management of Extractables and Leachables In managing leachables from rubber elements, it may be very important note the following: � Extractables can migrate into both liquid and solid merchandise (powder, lyophilized). Therefore, leachables found could differ in id from these used in the rubber formulation recipe. In dealing with extractables and leachables, there are two main variations between glass and rubber. The percentage of every raw material could differ barely, however the supplies themselves are fairly uniform. The second distinction is that tumbler compositions are normally not proprietary and are readily shared by the provider with the drug packager. These variations make the identification and quantification of glass extractables and leachables a lot easier than in either rubber or plastics. Glass has been used as a pharmaceutical packaging part for a number of hundred years, notably through the twentieth century through the tremendous growth of pharmaceutical business. Even though many predicted doom for the glass business when modern plastics grew to become out there some 50 years ago, using glass for pharmaceutical containers has endured. Impermeable to gases Easily cleaned, sterilized, and depyrogenated Transparent Rigid, strong, dimensionally stable Composition of Glass Components Commercial glass is an inorganic product of fusion that has cooled to a rigid state with out crystallization [51,52].
Generic kamagra soft 100 mg overnight delivery
Method parameters erectile dysfunction treatment cream kamagra soft 100 mg purchase free shipping, typically expressed in seconds young erectile dysfunction treatment generic 100 mg kamagra soft amex, dictate the period of every aforementioned cycle step. Parameters include time to attain preliminary goal vacuum, equalizing time, and vacuum loss take a look at time. Optimization of those take a look at methodology parameters maximizes check technique sensitivity for each product-package. In abstract, vacuum decay is a speedy, noninvasive, and nondestructive leak test technique. Depending on the check system, defects as small as 3 �m in effective diameter are reliably detected in a big selection of nonporous, inflexible packages. Test strategies are appropriate as a stability program integrity check or as an in-process check of clinical or industrial manufacturing lots. Larger scale, online equipment may be used for one hundred pc production lot testing, although leak check sensitivity is considerably lower than for the most sensitive off-line instruments. As vacuum decay depends on gas or liquid move through a defect pathway to facilitate detection, this clogging impact can have severe implications for sensitivity and precision of a vacuum decay leak check method, especially if carried out over a duration of time as could be carried out in a stability study. On the opposing aspect, a floor electrode collects current conducted by the package deal. Any liquid of greater conductivity than the bundle material present at or near a leak path situated close to the detector will cause a drop within the Applications As mentioned within the take a look at method selection portion of this chapter, each check know-how has a set of strengths and weaknesses. As such, vacuum decay is finest utilized to rigid or versatile nonporous package techniques containing stable product, although there are exceptions. Consideration could also be given to take away the first package from the system meeting for evaluation, though system disassembly procedure would require validation to affirm the method could be reliably and repeatedly carried out without damage to the first packaging. Positive electrical present occurred close to a laser-drilled hole within the glass barrel wall. Laserbased headspace utilizes a diode laser to emit wavelengths in the purple and near-infrared regions of the electromagnetic spectrum, where molecules similar to oxygen and moisture absorb mild. The underlying principle of laser absorption spectroscopy is that the amount of light absorbed by a molecule at a selected wavelength is proportional to the gas concentration and the gas stress. The laser passes by way of the gasoline headspace region of a sealed bundle; gentle is absorbed as a function of fuel concentration and pressure; the absorption data is processed utilizing phasesensitive detection methods; a mixer demodulates the radio frequency signal; and the output voltage, proportional to the absorption lineshape, is digitally converted and further analyzed by a microprocessor, yielding ultimate test outcomes. The whole space is proportional to the moisture partial pressure and focus. A variety of diode laser-based system configurations, both on-line and off-line, can accommodate process monitoring and management and/or inspection of particular person containers for oxygen, moisture, or vacuum. Voltage spikes can occur even if leak paths are clogged with dried product-an benefit not shared with pressurebased analyses that require mass flowing by way of an open leak path to enable leakage detection. Therefore, a quantity of detectors could also be stationed at various heights alongside a course of line to allow for analysis of the entire package deal. Laboratory-scale instruments and detectors that allow package rotation and whole package deal scanning are available, permitting for comprehensive evaluation of a person take a look at bundle. For instance, the utilized high-voltage present ought to be high enough to permit for adequate present to detect leakage paths but not too high that the present arcs over the package deal, on to the ground electrode, causing false-positive outcomes. Further concerns for methodology attributes are supplied within the Method Validation section of the present chapter. The frequency modulated diode laser output is converted to an amplitude modulation after passing through a gas pattern which absorbs at a specific wavelength. The amplitude modulation is proportional to gasoline concentration and may be part sensitively detected. The peak-to-peak amplitude of every spectrum is proportional to oxygen concentration. Subsequent measurements of unknown samples use this calibration information to convert measured absorption alerts into meaningful values of headspace gasoline concentration and/or gas stress. In the case of product sealed with an inert fuel overlay, leakage of oxygen into the container will be a function of diffusive flow pushed by the larger oxygen partial strain exterior the container. The results present that holes 5 �m will allow oxygen levels to rise above 1% inside 1 day; 2 �m holes will result in oxygen content material higher than 1% after about 8 days. Caution is advised, nonetheless, when attempting to predict package deal integrity for longer durations according to diffusion kinetics. Over time, packages are uncovered to pressure differentials from modifications in altitude or climate and even by doorways opening and closing, all of which drive faster, convective flux leakage, thus complicating such projections. Since the absorption power of water vapor is 1000X stronger than oxygen within the close to infrared, the total area of the absorption profile can be utilized to determine water vapor focus. In these scans, the entire space is proportional to the moisture partial pressure and concentration. In precept, any molecule undergoes pressure broadening and can be utilized for measuring the gasoline stress in a sealed container. These scans show how the absorption sign broadens as the total gas stress will increase from full vacuum to an intermediate stress and finally to atmosphere. Variations in element dimension, elastomer lubrication, gasoline flushing, stopper insertion, and even dealing with are only some of the components that may influence the finish result. Destructive testing for both oxygen content or vacuum degree utilizing different off-line take a look at methods is dear 522 Parenteral Medications the package is positioned into cryogenic storage, the fuel inside the vial shrinks while the exterior environment stays at ambient strain. This strain differential drives the ingress of dense, cold gaseous surroundings into the bundle through the fashioned breaches. When the vial is taken out of storage, nevertheless, and the elastomer is thawed above its Tg, the material will regain its viscoelastic properties, causing any shaped gaps to reseal and due to this fact trapping cold gasoline inside the vial. As the package deal conditions to ambient, the trapped dense headspace warms and expands, resulting in an over pressure inside the package which could be quantified by laser-based headspace evaluation. Tracer Gas Leak Test Methods-Vacuum Mode Leak detection by tracer gasoline analysis is a minimum of as delicate as laser-based headspace analysis. Helium is the commonest trace fuel used for bundle integrity testing, though hydrogen is also used (14, 15). Detection of helium by mass spectrometry is capable of detecting large leaks of 10 -2 Pa m3/s all the means down to ultrafine leaks as small as 10 -11 Pa m3/s. Helium hint gas testing is most useful for testing leaks in the average to ultrafine leak vary. Greatest sensitivity is possible using the vacuum mode, a deterministic test method that shall be discussed here. Tracer gasoline leak checks may be used within the sniffer mode which is probabilistic in nature and mentioned in a future section. In the vacuum mode, a helium-flooded sealed package deal is either totally enclosed in an evacuated take a look at chamber or the seal of the package is isolated inside a check fixture. There are attainable sources of error or method interferences unique to helium mass spectrometry. Steps to forestall elevated helium ranges within the check space embrace proper ventilation, remote helium cylinder location, and proper sample isolation fixturing. Helium also easily permeates by way of many materials, especially plastics and some elastomers. Thus, helium permeation via the check package deal must be characterised to stop misinterpretation of results.
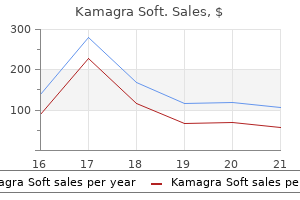
Discount kamagra soft 100 mg fast delivery
Disposable bags are at the center of the system as a result of the drug substance or product has the greatest holding time in these containers erectile dysfunction 37 years old kamagra soft 100 mg fast delivery. Bags that vary in size from lower than 100 L to greater than 1 erectile dysfunction treatment on nhs buy kamagra soft 100 mg mastercard,000 L are used in upstream and downstream bioprocessing and in fill/finish operations, together with media preparation, as bioreactors, and for sampling, and for storage and transportation. Consequently, multilayer bags are sometimes utilized in many purposes so as to preserve product integrity. These baggage are constructed to present fuel and moisture barrier properties, performance after sterilization, sturdiness, and biocompatibility (Table 23. The Flexel 3D movie manufactured by Sartorius Stedim Biotech consists of five layers. This building not only addresses issues in phrases of gasoline and moisture change but additionally supplies the movie with superior physical energy [136]. An array of various checks are utilized to optimize the bodily efficiency of single-use baggage. In addition, a water-burst check utilizing mannequin luggage and worst-case application testing of two-dimensional rockingmotion cell culture baggage and three-dimensional stirred bioreactor bags had been used to affirm the robustness of Flexsafe bags in several purposes. Disposable bioprocess luggage are commercially out there in various codecs and capacities and created from completely different supplies with a diversity of designs to go well with different purposes [138]. Many elements subsequently have to be considered when choosing an acceptable disposable bag, together with whether the plastic polymer is secure and suitable with the bioprocessing fluid. This information, in combination with data of the drug substance or drug product processing which will embrace processing volumes, chemical stability, compatibility, variety of campaigns, formulation components, processing conditions corresponding to temperature and stress, and most significantly, extractable and leachable considerations, can provide insights into the choice of a disposable bag for bioprocessing. Chemical Resistance Study Chemical compatibility studies must be performed to evaluate the choice of a single-use container previous to its selection. The checks should assess the potential chemical attack by the assorted solvent techniques together with buffers, natural solvents, or different parts which could be intended for drug product improvement. Typical additives utilized in movies designed for single-use bioprocess containers are slip brokers and antioxidants. Slip brokers, typically amides of fatty acids, are added to the polymer to present surface lubrication to the film throughout and after extrusion and to forestall singleuse container partitions from adhering to one another, thus reducing the stress on the movie during bag filling. Antioxidants are added to the formulation to inhibit degradation of the polymer by atmospheric oxygen. Oxygen will react preferentially with the antioxidant, limiting its interplay with the polymer chains and thus reducing the potential of polymer chain breakage. Thus, an evaluation of potential extractables is required for plastic disposable baggage to ensure compliance. Extractables are substances that can be extracted from a plastic utilizing solvent or extraction conditions anticipated to be more aggressive than the processing situations meant. The suppliers of the plastic bags or elements should provide a full and full potential extractable list which could be used to consider product suitability with the plastic disposable bag [145]. The examine confirmed how model solvents with extraction properties similar to these of the bioprocessing fluid or drug formulation might be used to achieve maximum overlap between extractables and leachables [142]. Protein Adsorption and Aggregate Formation Single-use techniques have become increasingly prevalent in downstream processing, final formulation development, and fill/finish of protein solutions. These techniques have additionally gained acceptance for processing and storage of recombinant proteins, including monoclonal antibodies, in both liquid and frozen states, at the manufacturing scale. However, the use of these systems raises the chance that container�protein interactions could affect the soundness of the biologic because of leaching of polymeric materials into the drug resolution or protein adsorption onto the plastic container floor. The major driving forces responsible for the nonspecific adsorption of proteins to surfaces are hydrophobic and electrostatic interactions. As mentioned on this chapter with regard to protein adsorption to surfaces of prefillable syringes and vials, one of many penalties of protein adsorption is its potential role within the formation of protein aggregates. It is therefore essential to develop applicable analytical methods to evaluate and characterize the appearance of protein particulates in drug options that come into contact with disposable bags. Sterile Barrier Integrity Maintaining barrier integrity is crucial to protecting the product from microbial contamination. When plastic baggage or components are supplied as sterile, the integrity of these merchandise have to be demonstrated. These exams may embrace helium leak testing, stress testing, dye ingress, or microbial ingress challenges. Extractables and Leachables Single-use bioprocessing bags and bioreactors have gained speedy acceptance by the biopharmaceutical industry as they offer a number of advantages over traditional stainless steel methods. However, there are vital issues raised by finish customers that the plastic supplies are extra susceptible to compound leaching, together with toxic substances, into the bioprocessing fluid that would negatively impact cell progress and product titers and potentially product high quality [141] and even compromise drug security when single-use baggage are used for intermediate or drug substance storage. These leachables are chemical substances that originate from the plastic supplies used to manufacture the baggage or are generated during gamma irradiation and storage of the polymer products. Additives are used for a quantity of causes, including to facilitate polymer processing and to improve the stability of the polymer to oxidation and gamma irradiation. Particulates While the benefits of single-use systems are clear, together with those benefits come new areas of concern related to polymeric materials and disposable methods. In addition to a consideration of leachables and extractables, protein adsorption, Plastic Packaging and the chemical and bodily properties of single-use methods is the problem of cleanliness. Because single-use systems are intended to be used "as is," the duty for his or her cleanliness has shifted from end users to distributors. The contaminants present in a system, similar to leachable substances or particulate matter, could additionally be transferred to the method media. The contaminants in upstream processes such as bioreactors and mixing techniques current much less of an issue because process fluids might be subjected to downstream filtration and purification steps and thus removed from the processing workflow. Thus, the final processing steps corresponding to drug substance storage and last filling are steps that demand the bottom levels of particulates and leachables in single-use techniques. The same concerns raised with regard to particulates in vials and prefillable syringes pertain to elements of single-use techniques together with tubing, fittings, and luggage. Ultimately, the primary purpose to higher understand and control particles in single-use systems is to scale back their medical impression and guarantee affected person safety. Infusion of particulate matter with a biotherapeutic can have quite lots of unfavorable penalties, similar to blood vessel blockage and pulmonary granulomas as properly as the event of an immune response to the drug which depends on a number of variables together with the amount, measurement, and shape of the particles; the route of administration; and affected person attributes similar to age and health standing [82]. In addition, as discussed on this chapter, there are also industrial penalties, including the necessity for heightened threat mitigation measures and inspections, the potential of reduced production yields and rejected batches of product, as nicely as potential product recalls and elevated scrutiny from regulatory bodies. These limits rely upon the method of detection used (light obscuration or microscopic particle count) and whether the sample comes from a large-volume (>100 mL) or small-volume (100 mL) container [147]. One current research examined bags made up of various materials to try to assess the potential contribution of single-use techniques to the overall particle depend in the drug product. The outcomes of this investigation suggested that particle ranges are dependent on the character of the material used to form the wetted surfaces of the bag in addition to the method of sterilization, with particulate ranges higher in bags that had been gamma-irradiated versus autoclaved [143]. In addition, particulates on the surface of the bag that arise from packaging and handling can doubtlessly contribute to the general particle rely.
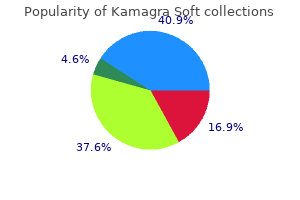
Kamagra soft 100 mg discount otc
It is essentially the most secure of the vegetable oils (except to light) as a result of it accommodates natural antioxidants diabetes and erectile dysfunction relationship order kamagra soft 100 mg online. Sesame oil has also been used to get hold of gradual launch of fluphenazine esters given intramuscularly [67] erectile dysfunction due to old age kamagra soft 100 mg generic with amex. In recent years, the use of injections in oil has diminished somewhat rather than aqueous suspensions, which typically have less irritating and sensitizing properties. Types of Vehicles Aqueous the vast majority of injectable merchandise are administered as aqueous options because of the physiological compatibility of water with physique tissues. However, any such additive can also produce unfavorable results corresponding to lack of drug solubility, exercise, and/or stability. No coloring agent may be added, solely for the purpose of coloring the finished preparation, to an answer intended for parenteral administration [1]. The reader is encouraged to check with a variety of publications that present comprehensive listing of formulation parts used in all marketed injectable merchandise [1,68�74]. Commonly used parenteral components and their ordinary concentrations are listed in Table 10. Pharmacopeias often specify the type and amount of additive substances that could be included in injectable merchandise. These requirements typically vary from compendia to compendia, so it is very important refer to the precise pharmacopeia that applies to the product in question. The properties and performance of those added substances shall be reviewed subsequent, except solubilizing agents and surfactant, which have been reviewed earlier. Buffers Maintaining the appropriate pH of the formulation is important for correct solubility and stability. Changes within the pH of a formulation could happen during storage because of degradation reactions throughout the product, interaction with container elements. Excellent evaluations on pH management within pharmaceutical techniques Formulation Development by Flynn [75] and Kaus [76] are beneficial to the reader. A suitable buffer system ought to have an enough buffer capacity to maintain the pH of the product at a stable worth throughout storage, whereas allowing the body fluids to regulate the pH easily to that of the blood following the administration. Tissue necrosis often occurs above pH 9, while excessive ache and phlebitis are skilled under pH 3. Parenterals administered by different routes are usually adjusted to a pH between four and eight. A suitable buffer system could be chosen from knowledge of a solubility/stability pH profile of the drug in resolution. By following the degradation over a given pH vary and plotting the rate constants versus pH, the pH of maximum stability (pH 6. In the case of procaine penicillin G, the solubility is lowest between pH 6 and seven, which is fascinating for the rationale that product is formulated as a suspension. Once the desired pH is set, a buffer system that provides adequate buffer capability could be chosen. The buffer capacity, is a sign of the resistance to change in pH upon the addition of both primary or acid substances and could be represented by the next expression: 171 where dB = change in concentration of base or acid, dpH = change in pH, C = molar focus of buffer system, and Ka = dissociation fixed of the buffer. A maximum worth at zero indicates that the best buffer capacity occurs at a pH equal to the = dB Ka H+ = 2. Buffer methods for parenterals usually include either a weak base and the salt of a weak base or a weak acid and the salt of a weak acid. The distance indicated by the arrows represents the efficient buffer range for each system, and the dashed traces symbolize the pKa for the system. The Henderson�Hasselbalch relationship is used to calculate the portions of buffer species required to present a desired pH: pH = pK a + log Csalt Cacid (10. Buffers can act as common acid or common base catalysts and trigger degradation of some drug substances. Such a mechanism happens with numerous amine and amine spinoff medication in methods containing polycarboxylic acids. The ionic energy contributions of the buffer system can also have an effect on each isotonicity and stability. Such reactions are mediated either by free radicals or by molecular oxygen and sometimes involve the addition of oxygen or the removal of hydrogen. For merchandise by which oxygen is instantly involved within the degradation, protection may be afforded by displacing oxygen (air) from the system. This is completed by bubbling nitrogen, argon, or carbon dioxide via the answer previous to filling and sealing within the last container. Drugs possessing a favorable oxidation potential shall be particularly vulnerable to oxidation. An enhance in hydrogen ion concentration causes a rise within the precise oxidation potential, E. In this equation, E zero is the usual oxidation potential, R the fuel constant, T the absolute temperature, and fixed 2 represents the variety of electrons participating in the oxidation�reduction reaction. Agents that have a decrease oxidation potential than the drug in query, and thus may be preferentially oxidized, are known as antioxidants. Therefore, morphine may be stabilized by reducing the pH or by including an antioxidant corresponding to ascorbic acid which will be preferentially and reversibly oxidized between pH 5 and 7. Ascorbic acid, in turn, can act as an antioxidant for hydroquinone as a end result of it has a lower oxidation potential and might be preferentially oxidized. Salts of sulfur dioxide, including bisulfite, metabisulfite, and sulfite, are the most typical antioxidants in aqueous solutions. Irrespective of which salt is added to the answer, the antioxidant moiety depends on the final concentration of the compound and the final pH of the formulation [83]. For example, in the presence of bisulfite, epinephrine types addition product as epinephrine sulfonate, which is inactive [85]. Ortho- or para-hydroxybenzyl alcohol derivatives similar to parabens react in a similar manner. Since small quantities (picograms) of barium or calcium could be extracted even from kind I glass, an insoluble sulfate can form within the solution [86]. Sulfite levels are decided 174 by the reactivity of the drug, the kind of container (glass seal vs. Another antioxidant, glutathione, an electron donor, stabilized the photooxidation of menadione, a synthetic analogue of vitamin K, by a charge switch advanced formation [87], thereby blocking the light-catalyzed oxidative chain reaction. Often a single antioxidant will not be sufficient to completely shield the product. Certain compounds have been discovered to act as synergists, growing the effectiveness of antioxidants, notably people who block oxidative reactions, for example, ascorbic acid and citric acid. While incorporating such antioxidants, the formulator must concentrate on their potential unwanted facet effects. Although, very broadly used, sulfites are related to several results upon parenteral administration, together with flushing, pruritus, urticaria, dyspnea, and bronchospasm [88]. In practice, the formulator can make the most of several approaches to shield the product from oxidative instability, corresponding to purging the solution and headspace with inert gas to exclude oxygen, lowering the pH, and addition of an antioxidant. One should ensure the utilization of high-purity excipients since trace impurities, particularly peroxides and metals, carried right into a formulation via ingoing elements, can also have a catalyzing impact on the autooxidation pathway. Well-protected, properly sealed packages that present a suitable headspace-to-product ratio also can provide some robustness to the product, thus making it much less sensitive to oxidation [89].
Buy discount kamagra soft 100 mg online
Concentrations between 400 and 650 mg/L are beneficial for efficient microbial inactivation and extra environment friendly gasoline removing from product at completion of the sterilant exposure erectile dysfunction treatment new delhi purchase 100 mg kamagra soft free shipping. The aeration areas ought to have the next capabilities: � Airflow detection alarms or indicators on the airhandling system to ensure steady operation � Recirculation erectile dysfunction drugs and glaucoma buy 100 mg kamagra soft otc. The microbial lethality of gamma rays and electrons is achieved by ionization; electrons are direct ionizing radiation, whereas photons are indirect ionizing radiation. The vitality transferred by these radiations through the sterilization process produces chemical and/or bodily adjustments at the molecular degree, resulting in chain scission, polymerization, cross-linking, sterilization, and disinfection. Physical Characteristics of Radiation By far, essentially the most commonly used of the three strategies is gamma radiation. Gamma rays are emitted from radioactive isotope supply supplies, the commonest being cobalt 60 (60Co). Having no electric cost or mass, photons transfer vitality to materials mainly through Compton scattering collisions with atomic electrons leading to a uniform, exponentially lowering depth dose distribution. The photon strikes free electrons within the materials and passes a half of their power to the electron as kinetic energy. These displaced electrons continue on their way, deflected from their original path. The scattered gamma ray carries the steadiness of the power because it moves off via the material, probably to work together once more with another electron. The parameters used to decide acceptable dose supply of gamma sterilization are as follows: � � � � Cycle time Product density Loading sample Density combine. It is the cascade of electrons that end result within the bodily and chemical changes in the materials as nicely as the destruction of microorganisms. Because the chance of Compton scattering is low, the primary beam of gamma rays will penetrate lengthy distances in material before the scattering happens. This means that the gamma rays deposit energy over a comparatively giant space in order that penetration is high (up to 50 cm), but the dose rate is low (Table 30. In distinction to gamma, electrons centered into a beam generated by a linear accelerator with beam energies of 5�10 MeV have each mass and charge, so they work together readily with other charged particles, transferring their kinetic energy to supplies by quite a few elastic and inelastic collisions. These interactions result in many directional adjustments, ionizations, and radioactive processes that sluggish the electrons and ultimately limit their penetration to only 5 cm into materials with a density of 1. E-beam vitality is subsequently deposited within materials over a short distance making the dose price very excessive (22,000 kGy/h for a 50-kW beam) allowing sterilization to take place in less than 1 min. The parameter measuring the energy transferred from the radiation source to the product is identified as the absorbed dose. The penetration of gamma rays and electrons is inversely proportional to product density. The absorbed dose is the amount of ionizing radiation power imparted per unit mass of a specified material and is expressed as the Gray (Gy) where 1 Gy = one hundred Rads or 1 kGy = 0. When a inhabitants of microbial cells is irradiated, the number of living items diminishes exponentially as the dose will increase, till no viable cells remain. The free radicals then react with the macromolecule altering its normal mobile metabolism, which outcomes in loss of the reproductive capacity of the microorganism. In a nonaqueous surroundings as present in sterilization of most medical merchandise, the principal sterilization mechanism Process reliability and consistency are assured by the wellknown decay fee of the radioisotope. When the supply and product are positioned accurately, small incremental modifications are routinely programmed into the timer setting to account for the decay, thereby allowing merchandise to be processed constantly. If product configurations stay the identical as validated, the only distinction in measured dose will be related to the variability in positioning product and uncertainties in dose measurement. The parameters used to decide acceptable dose delivery of E-beam sterilization are as follows: � � � � � � Beam power Beam present Conveyor speed Scan width Product geometry Product density. Process reliability and consistency are assured by management and monitoring of the beam, conveyor, and process parameters. Once parameters are established, merchandise will obtain the required dose as long as product density, product packaging, and orientation are unchanged. The change from one product to one other is comparatively easy for the explanation that results of the adjoining product are minimal. Dry heat kills the organisms by damaging oxidation of important cell constituents. Inactivation of essentially the most resistant spores by dry heat requires a temperature of about 160�C for 60 min. It can be used for anhydrous fat, oils, and powders which are impermeable to moisture. Moist heat kills organisms by coagulating and denaturing their enzymes and structural protein. Sterilization by moist heat of the most resistant spores usually requires 121�C for 15�30 min. Moist heat is used for the sterilization of tradition media, and all different materials by way of which steam can penetrate. Sterilization may be done at decrease temperatures in a given time at a shorter length at the similar temperature. Many sterilization cycles have been developed to be used in a moist heat environment with calibrated gear that has been properly installed and validated. Among the 686 processes commonly used in industrial moist warmth sterilization are the next: a. Gravity air displacement: Sterilizers use gravity to take away air from their chambers. Steam introduced into the chamber creates a layer above the air, which increases until the air is pushed down by way of a drain on the backside of the unit. After the air is eliminated, steam temperature and strain build, and exposure time begins when the sterilization temperature is reached. Gravity sterilizers are used to sterilize surgical instrumentation, liquids, and linen. Dynamic air elimination (Prevac): this process is meant to sterilize merchandise consisting of porous materials and/ or gadgets having cavities the place air is difficult to remove. Prevacuum sterilizers use a pump to remove air from the chamber before steam is introduced. Dynamic air elimination models are, therefore, extra efficient than gravity air displacement sterilizers as a outcome of air is pumped out before steam enters the chamber, so the steam can immediately penetrate packages. There are numerous available processes by which filtered compressed air is used to ensure that, for half or for the period of the sterilization cycle, the pressure on the skin of the product equals or exceeds the inside pressure. These processes embody cycles using air/steam mixtures, water spray, and water immersion. Steam sterilization requires four important parameters: steam, temperature, strain, and time. Dynamic air removal, washer sterilizers, and flash sterilizers require a temperature of 270�F�275�F (132�C�135�C). Note that because the psi required to reach sterilization temperatures is expounded on to the altitude, the precise psi required may differ slightly by geographical location. The choice of the suitable approach is based on the nature of the product, bioburden, and packaging, manufacturing situations, and kind of sterilization gear.
Buy 100 mg kamagra soft visa
Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of reducing binding constants erectile dysfunction treatment center order 100 mg kamagra soft fast delivery. A thermodynamic analysis of the binding interaction between polysorbate 20 and 80 with human serum albumins and immunoglobulins: a contribution to understand colloidal protein stabilisation erectile dysfunction and pregnancy buy kamagra soft 100 mg low price. In basic, the easiest way to treat ophthalmic ailments is with an area remedy similar to a topically administered eye drop. However, the biological design of the attention is optimized to hold the surface of the eye clear of all overseas substances and to provide a substantial barrier to the transport of supplies into or out of the eye. The ophthalmic formulator, therefore, should start with an excellent understanding of the physiology of the eye and perceive what ophthalmic drug supply prospects can be found. The formulator must also perceive the nature of the drug substance that needs to be delivered to the eye and its limitations. The final job of the ophthalmic formulator is to uncover the best way to bring the drug and the attention together in a fashion that will provide the optimum benefit to the affected person. This article will concentrate on the anatomy and physiology of the eye and the challenges in drug delivery to this organ. The objective might be to familiarize the reader with the biopharmaceutical aspects of drug supply to the attention, the varied methods for focusing on Development of Ophthalmic Formulations totally different tissues inside the eye, and to provide a guide to a rational strategy to formulation growth for ophthalmic drug delivery. We may also provide a short overview of ophthalmic formulation preservation, manufacturing and packaging, and regulatory pathways for bringing a formulation to market. Finally, the chapter will talk about some latest advances in drug delivery and the method forward for ophthalmic drug supply. In recent years, a larger understanding has evolved around the structure and composition of the tear film. Structure and Fluid Composition of the Eye the attention globe is frequently cleansed and hydrated by the secretions of the nasolacrimal system. The eye globe could be divided into three concentric tunics: the fibrous tunic (comprising the cornea and sclera), the vascular tunic (consisting of the iris, ciliary physique, and choroid), and the retinal tunic. Other inside elements of the eye embody the lens, aqueous humor, and vitreous humor. When a drug is delivered to the outer floor of the eye, it could have to diffuse via many of those tissues before it may possibly reach the target tissue. The following is a very basic evaluate of the main elements of the attention related to the drug delivery. Tear Fluid Secretion and Volume Tears are constantly secreted by the lacrimal glands and the goblet cells. When additional fluid is added to the attention surface, the eye can maintain about 25 L of fluid but will appear "watery" as a end result of the added liquid. With greater additions of fluid, the surplus tear fluid will instantly overflow at the lacrimal lake [1] or be splashed into the eyelashes by reflex blinking [3]. The meibomian secretions are primarily wax esters and sterol esters (about 59%), and phospholipids (about 15%), and the rest are diglycerides, triglycerides, free fatty acids, free sterols, and hydrocarbons [4,5]. The polar lipids (phospholipids) are primarily phosphatidylcholine (40%) and phosphatidylethanolamine (18%) [6]. The meibomian secretions are produced at a fee of about 400 g/h and are excreted onto the lid margin and the anterior surface of the tear film by Nasolacrimal System the nasolacrimal system consists of three components: the secretory system (lacrimal glands, meibomian glands, and goblet cells), the distributive system (eyelid actions and blinking), and the excretory system (lacrimal puncta; superior, inferior, and customary canaliculi; lacrimal sac; and nasolacrimal duct). The nasolacrimal system plays a serious role in protecting and hydrating the attention floor. It also has a significant influence on the quantity of drug absorbed to the attention from topical administration. The thickness of the oil film on the tear fluid has been measured by numerous interference strategies giving values of ranging from 20 to a hundred and sixty nm in thickness [5,7]. From this thickness, the steady-state amount of oil present on the floor of the tear fluid is calculated to be about 9 g per eye [5]. Parenteral Medications fluid, and the levels of calcium and magnesium are less than 1/200th of the sodium levels [3]. Higher-than-normal osmolality in the tear fluid is often seen in patients with dry eye syndrome. Abnormally high evaporation of tear fluid increases the salt ranges and ends in higher osmolality. As a end result, many products for the remedy of dry eye are often formulated with lowerthan-normal osmolality. Tear Fluid Proteins and Enzymes Tear fluid accommodates proteins in excessive concentration, about 13. Major elements embrace lysozyme (an antibacterial enzyme), lactoferrin (which sequesters iron), secretory immunoglobulin A (an antibody important for mucosal immunity), serum albumin, lipocalin, and lipophilin [9]. In addition, more than 400 different proteins have been recognized that serve varied roles within the tear fluid [9]. Tear Fluid Viscosity and Surface Tension the viscosity of the tear fluid is primarily controlled by the upper molecular weight proteins dissolved in the lacrimal fluid. The viscosity of human tears has seldom been decided as a outcome of the difficulty of accumulating sufficient pattern for a determination. The viscosity of ophthalmic solutions may be elevated in an effort to improve retention on the ocular surface. The floor pressure of the tear fluid depends on the presence of soluble mucins, lipocalins, and lipids. Tear Fluid Mucus Layer the mucus layer is secreted onto the attention floor by the goblet cells. Mucus layer consists of glycoproteins, proteins, lipids, electrolytes, enzymes, mucopolysaccharides, and water. The mucus layer varieties a gel layer with viscoelastic properties, which protects and lubricates the attention. The mucus layer might hinder drug supply by forming a diffusional barrier to macromolecules, however it might additionally bind different substances. Holly demonstrated that the unfold of lipids on the floor of the eye is facilitated by mucins [11]. Fibrous Tunic Cornea the cornea is a clear structure answerable for the refraction of light entering the attention. The epithelium-a stratified squamous epithelium made up of five layers of cells (ten layers on the corneoscleral junction, i. The epithelial cells are linked through tight junctions that restrict drug permeability considerably. The stroma-accounts for 90% of the cornea thickness and is principally composed of water and collagenous lamellae that offers the strength and structure for this layer and yet allows the penetration of light. The primary buffering components present within the tear fluid are bicarbonate and proteins [12,13]. Rather, the tear fluid has more than twice the buffer capacity to resist drops in pH than it has to resist will increase in pH [13]. As a results of this uneven buffering capacity, unbuffered solutions within the pH vary of 4. Solutions with higher buffer capacities, significantly if higher than that of the tear fluid, may be uncomfortable to the eye if they end in a major shift of the tear fluid pH. Tear Fluid Osmolality the tear fluid osmolality normally ranges from about 300 to 320 mOsm/kg [3,12], and many of the osmolality in the tear fluid can be attributed to the salt content of the lacrimal fluid, which is primarily sodium chloride, sodium bicarbonate, potassium chloride, calcium chloride, and magnesium chloride [3].
Cheap 100 mg kamagra soft free shipping
Written procedures for sterilization process operations erectile dysfunction pump implant video kamagra soft 100 mg purchase, or reference to particular operator manuals four erectile dysfunction see urologist buy kamagra soft 100 mg with amex. List of all process parameters with set factors and minimum and most tolerances, and reference to the recording and controlling instruments for every 7. Requirements for routine high quality management checks and periodic audits related to sterilization 8. Written criteria for sterile product acceptance, reprocessing, rejection, and release for distribution, including instructions for choice, handling, and testing of samples. The high quality function is normally answerable for reviewing sterilization documentation. Even if the method is carried out by a contractor, the manufacturer is answerable for assuring that the appropriate cycle was carried out and that the cycle parameters had been within acceptable tolerances established through the validation, as follows: 1. Parenteral Medications specification must describe the aspects of the sterilization course of essential to guarantee conformance with the validated dose and dose mapping and be maintained with a longtime change management procedure. Written procedures for sterilization process operations, or reference to particular operator manuals 5. Sterilizer tote loading configurations and dose mapping displaying relationship between the reference level and the maximum and minimal dose positions 6. Specified minimum dose and minimal and most tolerances, and reference to the dosimeter system used routinely eight. Requirements for routine quality management exams and periodic audits related to sterilization 9. Failure to meet the bodily specification ought to end in quarantine of the sterilization load and in an investigation. Since radiation results on materials are cumulative, any determination to resterilize have to be primarily based on acceptable product aging take a look at information after multiple sterilizations. Process interruptions or delays ought to be evaluated to determine the impact on the microbiological high quality of the product and on the dosimetry methods. In addition, to ensure the numbers and resistance of the bioburden remain steady, a verification dose audit is performed each quarter. This is basically a repeat of the initial validation dose experiment however solely ten bioburden samples are pulled from a single lot. The result of the bioburden check is for information solely as a end result of an additional ten merchandise are dosed on the authentic validated verification dose no matter the current bioburden ranges. Routine Monitoring for Radiation After successful completion of the irradiation sterilization validation, a process specification must be written which explains the correct procedures to be adopted routinely. It may be the oldest method in use with the primary practical sterilizer dating to the late 19th century [1]. The huge trade experience with steam sterilization has resulted in the improvement of a wide selection of steam processes tailored for specific purposes. In every of those, the sterilization course of is achieved by the presence of liquid water on the floor of the microorganism at elevated temperatures [2]. The liquid water is critical for coagulation of proteins inside the microbe that end in its dying and is implicit in "moist heat" sterilization. The requirement for liquid water must not be overlooked; sterilization with superheated steam (where no liquid is present) has much more * Sterilization within the absence of liquid water (dry heat) requires substantially greater temperatures (typically >150�C) and impacts the microorganisms very differently. Moist-heat sterilization may be accomplished along the saturation curve itself where water is current both as liquid and as a gasoline (saturated steam) or within the liquid region (above the saturation curve) where the stress exceeds saturation, and only the liquid phase is current. Sterilization with saturated steam is preferable to water, as a end result of the extra heat available when the saturated steam condenses and releases its latent heat of condensation. The rapid transfer of heat to the gadgets to be sterilized by condensing steam is crucial to fast destruction of microorganisms and a serious cause moist warmth is most popular over other sterilization strategies. Microbiology of Sterilization the death of microorganisms by all sterilization methods shares a typical phenomenon [3]. The log variety of surviving microbes A 1�C drop in temperature for 1 g of liquid water releases 1 calorie. The condensation of 1 g of saturated steam to liquid water at 121�C releases approximately 525 calories. The steeper the slope of this line, the much less resistant the organism is to sterilization course of. The D-value can be influenced by a number of components aside from the microbial id including restoration media, age of the microbe, recovery methods, substrate on which the microbe is uncovered, etc. The D-value is defined because the time in minutes to scale back the microbial inhabitants by 1 logarithm or 90%. The inverse of this slope is called the D-value and is often expressed in minutes. Steam Sterilization larger than vegetative cells under the typical moist-heat course of situations [2]. The body of knowledge regarding steam sterilization and consistency of the results is such that mathematical correlations between the physical and microbial information are utilized to provide acceptable course of management [2]. These correlations are essential to assure product security (sterility) to the desired degree. The dotted line portion of the death curve represents the chance of surviving microbes where their quantity is just too low to depend. Were the process to be operated at precisely the conditions where the D-value has been determined, initial validation 697 and day-to-day process control would be greatly simplified. Unfortunately, this may be very difficult to present constant process conditions (fixed temperature for the entire sterilization cycle) in routine sterilization process on a business scale. There are many real-world factors that forestall fixed circumstances in production settings together with chamber size, load size, merchandise complexity, merchandise wrapping supplies, and merchandise orientation. To accommodate these components, a means for relating physical conditions at various temperatures to microbial destruction is critical. The D-value, which is basically the rate at which microorganisms are killed, is predominantly a operate of the temperature-the greater the process temperature, the extra speedy the destruction of microorganisms. The General Method for sterilization course of analysis uses this temperature dependency to allow for the estimation the lethal impact on microbes at a variety of temperatures close to the D-value [6,7]. The General Method estimates the lethality over the process period by calculating the kill rate for microorganisms because the temperature progresses by way of the sterilization cycle. When first developed, the concern was meals security and the potential survival of Clostridium botulinum in canned meals. A course of temperature of 250�F was discovered to be efficient for this process, and this situation was established as the standard base temperature for estimation of sterilization course of lethality. Simple mathematics can be utilized to calculate estimated lethality at different temperatures utilizing the lethality equation. For parts sterilization, it begins during the come-up and ends when evacuation of the chamber begins at the conclusion of the dwell interval. It is customary to only contemplate lethality contributions at temperatures above 100�C as a end result of the lethality contribution under that temperature is miniscule [8, 9] (Table 31. Probability of a Non-sterile Unit the period of sterilization process is chosen to ship lethality enough to confidently destroy the microorganisms present.
Kamagra soft 100 mg buy without a prescription
The vial containing the lyophilized drug could be attached to the bag and reconstituted by transfer of the diluent to the vial best male erectile dysfunction pills over the counter kamagra soft 100 mg buy on line, and the drug solution is then drawn again into the bag for administration erectile dysfunction protocol by jason 100 mg kamagra soft buy with amex. Dual-chamber techniques consist of a single unit with the lyophilized drug and the diluent in separate vials or cartridges. Reconstitution is performed by pushing in the syringe plunger to pressure the diluent via a channel leading to the second vial containing the drug. The reconstituted drug can then be transferred using a needle or via a luer connection. Innovation within the space of novel reconstitution methods has been driven by the necessity to enhance the effectiveness of the reconstitution process and to make it safer, thus enhancing affected person compliance with the dosing routine by lowering the potential for accidents because of unintentional needle stick and drug spray-backs. Consequently, human elements engineering has become a key 487 side of system improvement. Safety options such as automatic needle retraction for disposal of those techniques, for example, have made them extra convenient to use. By offering the system and the drug in a single kit, pharmaceutical firms can differentiate their product in the market, and use of those systems eliminates overfill of the drug as only the required dose must be included. One market study has identified roughly 50 gadgets, a significant number of that are dual-chamber or different novel techniques, which have been developed to reconstitute medication [24]. Most of those are disposable and are designed for injectables somewhat than infusion medicine. Primarily made up of plastic, reconstitution units are sterile, non-pyrogenic, biocompatible, and totally supported by appropriate regulatory filings [27]. The established producers in this area include Becton Dickinson, West, Vetter Pharma, and Ypsomed, but smaller corporations corresponding to Sensile Medical and Integrity Bio have entered the field to benefit from rising alternatives. Plastic vial adapters can present protected, straightforward to use, and cost-effective diluent switch to a vial containing the lyophilized drug. The adapter snaps onto the neck of the usual vial after the plastic button has been removed, and a plastic spike pierces the stopper, eliminating the use of a needle. This is a perfect answer for connecting vials of different sizes and likewise eliminates the usage of a needle. These superior plastic reconstitution techniques developed by Medimop Medical Projects, Ltd. They additionally can be utilized with in-line filters and will assist drug manufacturers scale back residual quantity and the amount of overfill within the drug vial [27]. Parenteral Medications molds, the place the tube-like parisons, or plenty of molten plastic, descend through gravity. When these reach the required size, the primary stage of the mold closes around the parisons that are then fashioned into the physique of the vial. The mould enters a fill zone the place tubes descend into the newly fashioned vials to dispense the liquid drug product into each vial. After vacuum tubes place sterile stoppers into the vials, the vials are sealed and transported out of the machine for secondary processing. The final is achieved by reducing particles, course of steps, and human interplay and by introducing measures to enhance air quality. Plastic molding and packaging in environmentally managed clean rooms often produces containers which have a really low bioburden and a low degree of particulates. To minimize scratching, care is usually taken to not stack vials too tightly during processing. Vial spacing in the course of the autoclave sterilization process in addition to slow cooling and depressurization of the autoclave may assist mitigate this effect [29]. Sterile vials or containers are non-pyrogenic, have a very low particulate degree, and might be used to store and transport drug merchandise as early as first-in-human research. During the filling of plastic vials, care ought to be taken to cover information rails and vial handling change components with a material that will restrict scratching of the vials. In addition, the velocity of the filling line might have to be adjusted to enable filling of the lighter plastic vials. Prefillable Syringe Systems Market Considerations In the current world market, more than three. Today, the overwhelming majority of biologics are delivered in injectable codecs, and throughout the top 100 prescription merchandise in 2020, organic products are anticipated to account for more than 50% of gross sales [33]. Drug and system developers are additionally pursuing novel noninvasive methods of parenteral drug supply, and more of those needleless injection devices are being launched into the market. Once a niche market product restricted to a few vaccines and anticoagulant therapies, the prefillable syringe market has now mushroomed because of a combination of many components [35]: � the extraordinary progress of therapeutic biologics. However, plastic prefillable syringes continue to make advances, especially the place glass has been proven to be unsuitable as a delivery system, and the proportion of plastic-based prefillable syringes is expected to improve by 5% between 2015 and 2020 [35]. These syringes are available sizes between 2and 50 mL and include generally used generic medicine similar to heparin, atropine, and epinephrine in small volumes, and electrolytes similar to sodium bicarbonate and saline in bigger volumes. More specialised syringes ranging in measurement from 50 to one hundred twenty five mL have been developed for use with X-ray contrast media that are dosed by X-ray energy injectors for procedures similar to computed tomography imaging and cardiovascular angiography [36]. In simply over the last decade, pharmaceutical drug merchandise have been permitted to be used with prefillable plastic syringes, together with a new chemical entity for oncology and a peptide drug product for the remedy of osteoporosis (Table 23. Although not reaching the adoption level of glass syringes, plastic syringe systems proceed to draw interest from pharmaceutical manufacturers due to current enhancements in design, composition, manufacture, and performance. Multiple vendors supply different sizes of syringes in sterile nested configuration or non-sterile bulk packaging. Plastic syringes are sterilized by either autoclave, radiation (gamma or e-beam), or ethylene oxide, but not by dry warmth, and are provided as assembled sterile syringes that are prepared for filling. The molding process additionally offers a larger degree of flexibility to embody design features corresponding to a plastic finger grip that can be combined with a backstop to prevent the piston from being pulled out of the barrel. There are also a variety of unbranded cyclic olefin prefillable syringes presently obtainable. This therapy imparts the barrier properties and other advantages of glass with the advantages of polymer systems all throughout the same gadget. Process Considerations Plastic prefillable syringes at the moment are available in sterile and ready-to-use formats. Ready-to-use syringes are positioned into a polymeric nest, and the nest is ready in a plastic tub covered by a Tyvek inlay and sealed with a Tyvek seal. This unique mixture of properties makes Tyvek light-weight and powerful, vapor permeable however moisture and chemical resistant, as nicely as puncture, tear, and abrasion resistant. Tyvek is also opaque and low linting to reduce the risk of particulate matter contamination. As glass prefillable syringes are already being crammed formatted in tubs, the transition to plastic prefillable syringes in an identical tub and nest configuration has been achieved utilizing the identical filling machines, and most industrial filling organizations can accommodate plastic prefillable syringes. There are, nevertheless, certain bodily differences between glass and plastic that must be thought of before running plastic prefillable syringes on a filling/processing line designed for glass prefillable syringes.