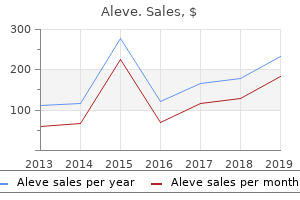
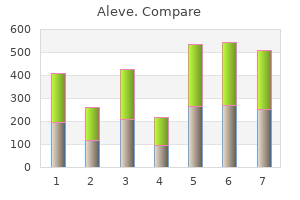
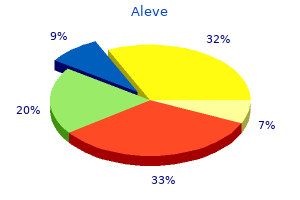
Aleve
"Discount 500 mg aleve overnight delivery, treatment for pain related to shingles".
By: L. Yasmin, M.A.S., M.D.
Co-Director, Washington State University Elson S. Floyd College of Medicine
A positive response to an elimination diet should not be construed as a definitive diagnosis unless there is compelling supportive evidence regarding specific foods pain treatment center in lexington ky buy aleve 250 mg without prescription. Another use for an elimination diet is to establish baseline status before undertaking oral food challenges; the response to oral food challenge is potentially definitive but must be performed for each food under consideration natural pain treatment for dogs purchase 250 mg aleve otc. Therefore prescription pain medication for shingles order aleve 250 mg without a prescription, procedures to reduce this possibility need to be implemented pain treatment center mallory lane franklin tn order aleve 250 mg amex, such as masking the challenge substance (blinding) and using placebos. The format of a food challenge can be applied to evaluate any type of adverse event attributed to foods due to both allergic and nonallergic hypersensitivity mechanisms. The challenge procedure, its risks, and its benefits must be discussed with the patient and/or the caregiver. Several factors are considered, including the evaluation of the likelihood that the food will be tolerated, the nutritional and social need for the food, and ability of the patient to cooperate with the challenge. In limited circumstances, the food could be administered with potential adverse reactions monitored at home by the patient and parents. On the other hand, if there is a reasonable potential for an acute and/or severe reaction, or if there is strong patient anxiety, physician supervision is recommended. Except in the uncommon circumstances described previously, oral food challenges are undertaken under direct medical supervision. A risk evaluation must be made regarding location of challenge (office, hospital, intensive care unit) and preparation (eg, with or without an intravenous line in place). Although generally considered a safe procedure when undertaken by qualified personnel, it must be appreciated that oral challenges can elicit severe, anaphylactic reactions, so the physician must be immediately available and comfortable with this potential and be prepared with emergency medications and equipment to promptly treat such a potentially life-threatening reaction. Although the open challenge is most prone to bias, it is easy to perform since no special preparation is needed to mask the food. Bias becomes an issue when the challenge food causes symptoms, particularly subjective ones. Therefore, open challenges are a good option for screening when several foods are under consideration, and if a food is tolerated, nothing further is needed. If there is a reaction to an open challenge used in the clinical setting, and there is concern that the reaction may not have been physiologic, the format could be altered to include blinding and controls. False-negative rates for double-blind, placebo-controlled food changes are low (usually 3%). The diagnostic utility of a test in regard to making a diagnosis in an individual patient is influenced by (1) the possibility of the disease existing in the individual being tested (prior probability) and (2) the characteristics of the test itself (sensitivity, specificity). To determine prior probability, it is necessary to undertake a careful history and to understand the epidemiologic features of food allergic disorders. Description of the latter is beyond the scope of this Practice Parameter but may be found in reviews959,986,987 and the Food Allergy Practice Parameter. Many studies use open food challenges with objective symp- toms as an end point, or sometimes they rely on convincing clinical histories. Conversely, progressively lower levels of food specific IgE (reflected by smaller skin test results or lower serum test results) are associated with a better chance to tolerate the food. As more studies emerge comparing serum and prick/puncture skin test results with clinical outcomes in wider age groups and populations with various disorders, further conclusions of test utility will be possible. In regard to skin prick testing, various reagents (fresh food, commercial extracts) and techniques of testing (probe type, location on the body, method of measurement, timing of measurement) are variables that affect final results and are additional obstacles in regard to applying study results to a particular patient. Similarly, a wheal size of 3 mm to peanut in children with atopic dermatitis was associated with a positive predictive value of 61%, whereas the same wheal size had a positive predictive value of 28% in children at low risk according to their clinical histories. A means to apply prior probability and test results in a particular patient to improve diagnostic accuracy is through the use of a calculated likelihood ratio. The likelihood ratio is simply the ratio of the odds that the patient whose test results fall within a particular range has the disease divided by the odds that they do not.
Please be advised that the substance of the comments and the identity of the individuals or entities submitting the comments will be subject to public disclosure pacific pain treatment center victoria bc discount 250 mg aleve free shipping. The proposed rule would also make changes to E&T components including: Replacing job search with supervised job search as a component; eliminating job finding clubs; replacing job skills assessments with employability assessments; adding apprenticeships and subsidized employment as allowable activities; requiring a 30-day minimum for provision of job retention services; and allowing those activities from the E&T pilots authorized under the Agricultural Act of 2014 (Pub treatment for residual shingles pain buy aleve 250mg with visa. The proposed rule would also require that dfw pain treatment center & wellness clinic cheap 500mg aleve with amex, in addition to providing one or more E&T components pain medication for dogs dose discount aleve 250mg overnight delivery, all E&T programs provide case management services to E&T participants. It would also establish a funding formula for reallocated E&T funds, and increase the minimum allocation of 100 percent funds for each State agency to $100,000, as prescribed by the Act. The proposed rule would require State agencies to re-direct individuals who are determined illsuited for an E&T program component to other more suitable activities. The proposed rule would add the requirement that all State agencies advise certain types of households subject to the general work requirement at recertification of employment and training opportunities. The rule would also require State agencies to provide to all households subject to work requirements with a consolidated written statement and comprehensive oral explanation of the work requirements for individuals within the household. The proposed rule would allow for more evidence-based components and require more accountability on the part of both State agencies and E&T participants while also retaining State flexibility. As a result, the Department proposes several changes to the way E&T programs are described. Under the proposed rule, an E&T program would be defined as a program providing both case management and one or more E&T components. The Department will discuss each of the proposed regulatory changes in more detail below. The Department instructed State agencies in the March 6, 2019, Informational Memorandum on Farm Bill E&T. The Department encourages State agencies to take full advantage of the workforce development expertise that already exists in their States to inform their E&T programs. Generally, E&T programs must be delivered through statewide workforce development systems-a broad network of service providers which may include: Government and the public sector; community-based organizations and nonprofits; employers and industry; occupational training providers; and post-secondary institutions, such as community colleges. State agencies should work with their Departments of Labor, State and local workforce development boards, and American Job Centers, as well as tribal workforce development programs, to obtain comprehensive labor market information when designing their E&T programs, and to capitalize on existing workforce development infrastructure and resources to ensure E&T participants have access to appropriate E&T services necessary to move them into good jobs and toward economic self-sufficiency. The Department encourages State agencies to design programs that are responsive to the needs of employers. Local Departments of Labor or American Jobs Centers may have existing relationships with local employers through which they have generated important information about the local labor market and employer training needs. State agencies should leverage the insights gained through these existing relationships as they build their own E&T programs. The Department seeks comments especially from State agencies and E&T providers on the ways in which this provision can best be implemented by State agencies choosing to provide supervised job search as a tool to move E&T participants toward improved employment outcomes. What criteria would State agencies consider when determining State-approved locations. How would these different approaches affect the ability of E&T participants to access supervised job search activities and State agency administrative burden What types of activities would State agencies include as part of this supervision. How might different approaches impact E&T outcomes and move participants toward self-sufficiency through work What modes might State agencies consider to track the timing and activities of participants. How would these different potential approaches affect the ability of E&T participants to access supervised job search activities and State agency administrative burden In addition, the Department seeks comments describing current job search programs operated as part of E&T programs or other workforce development programs that are directly supervised and where the timing and activities of participants are tracked by the State agency or providers. How are State agencies or providers providing this direct supervision and tracking the timing and activities of E&T participants Do these programs require that the activities and supervision take place at a State-approved location
When the microflora balance in the gut is disturbed pain after treatment for uti aleve 500mg without prescription, potentially pathogenic clostridia begin to produce toxins and proteolytic enzymes sciatica pain treatment guidelines discount 500 mg aleve. Factors involved in microflora disturbance include: intestinal infections (eg coccidiosis) pain treatment centers of america little rock 250 mg aleve, nutritional factors (protein source pain treatment winnipeg 500 mg aleve fast delivery, grain source, diet changes), management: type of litter, timing of feed changes, antibiotic treatments. Although it can be seen at any age, the acute clinical form is primarily a disease in young chickens, showing severe depression, reluctance to move, diarrhea, ruffled feathers and sudden death and increased mortality. In the clinical form the necrosis might progress into a fibrinonectoric enteritis forming a diphtheritic membrane. In the mild form, focal areas of intestinal mucosal necrosis without further clinical signs can be found. Transmission Clostridium perfringens is an ubiquitous organism that can be found in faeces, soil, dust contaminated feed and litter. Diagnosis Clinical signs in combination with typical gross and microscopic lesions and isolation of the causative agent will confirm the clostridial infection. Treatment antibiotic 80 81 Miscellaneous Bacterial Diseases Necrotic Enteritis Miscellaneous Bacterial Diseases Necrotic Enteritis Control Vaccination of breeders with inactivated vaccines based on toxins inducing active and passive immunity have shown to offer good protection. Maintain microflora balance with management of all related factors; management, coccidiosis control and nutritional factors. Infected breeder hens may transmit the disease agent vertically to their offspring. Isolation of the organism and its biochemical determination may be attempted but care should be taken that these are carried out using appropriate methods in order to avoid unreliable results. Differential diagnose with bacteria causing similar disease patterns is recommended (E. The birds may show respiratory disease with watery eyes and swelling of the sinus infraorbitalis. A severe purulent pneumonia, accompanied by airsacculitis and pericarditis may be found in broilers as well as in turkeys. Treatment Infections are normally treated with broad-spectrum antibiotics with variable degrees of success. Control An inactivated vaccine for broiler breeders is available to prevent the disease in the vaccinated birds and provide maternal antibodies to the offspring of the vaccinated breeders. Fowl typhoid is caused by Salmonella gallinarum, which is related to , but not identical to S. Treatment Treatment with antibiotics of pullorum/fowl typhoid disease will not cure but reduce clinical signs and is undesirable from a standpoint of eradication. It is far more practical to control the disease by elimination of infected carrier breeder hens. Blood testing (monitoring) of breeder chickens by the serum plate or tube agglutination test with suitable S. Such control measures will stop the incidence of egg-transmitted pullorum disease/fowl typhoid. If hatching eggs from tested pullorum-free breeders are kept free from contamination through infected eggs from infected breeders or through contaminated equipment, chickens can remain carrier after treatment. Transmission Pullorum and typhoid can be transmitted horizontally and vertically by infected (carrier) breeder hens through their eggs. Chickens that hatch from such infected eggs will have typical pullorum disease (white diarrhoea) and high mortality. Fowl typhoid is more a disease of adult chickens, with high mortality and morbidity. Horizontal transmission is important with fowl typhoid through infected droppings, dead bird carcasses, and infected clothing, shoes, utensils and other fomites. Other birds such as quails, pheasants, ducks, peacocks and guinea fowl are susceptible. Morbidity and mortality can be highly variable (mortality can reach 25-60%) Lesions; acute phase septicaemia-enlarged and congested liver, spleen and kidneys, pericarditis. Transmission Direct transmission by infected water, feed, or droppings has been proved.
This section discusses a rationale for therapeutic apheresis use in the disease and summarizes the evidence in this area elbow pain treatment exercises purchase 250 mg aleve free shipping. This section briefly describes technical suggestions relevant to the treated disease pediatric pain treatment guidelines aleve 500 mg, which the committee believed were important to improve quality of care or increase chances of a positive clinical outcome pain management treatment center generic 500mg aleve amex. Not all diseases may have specific technical notes; in such instances georgia pain treatment center generic 500mg aleve with mastercard, a general statement referring to the introductory text is provided. However, in some settings, due to significant variability in treatment schedules reported by different groups, the committee suggested what is believed to be the clinically most appropriate frequency. Application of this information may vary depending on the patient and clinical presentation, and is left to the discretion to the treating physician. Terms such as plasma or albumin were used to denote the type of replacement fluid. In some instances, the number of procedures/series which may be reasonably employed in the particular clinical situation is suggested based upon currently available data. The committee believes that a thoughtful approach to patient management is required to establish reasonable and scientifically sound criteria for discontinuation of treatment. For additional information, one textbook in the field of apheresis medicine which users of the Special Issue may find useful is Apheresis: Principles and Practice, Third Edition (McLeod, 2010). In Table 7, we propose information that may be included in a consultation note before performing an apheresis procedure. This standard approach to consultation may be particularly helpful to readers who may have limited experience in the field of apheresis medicine. As with previous editions, there is a significant expansion in the number of indications (relative to the number of diseases categorized) and is accounted for by some diseases having several categories and recommendation grades due to multiple indications within the same disease, or multiple apheresis modalities used to treat the same disease with different grade recommendations. This determination should be made using appropriate medical judgment through consultation between the requesting physician and the physician administering apheresis. In some General Issues to Consider When Evaluating a New Patient for Therapeutic Apheresis Description Based on the established/presumptive diagnosis and history of present illness, the discussion could include the rationale for the procedure, brief account of the results of published studies, and patient-specific risks from the procedure. The effect of therapeutic apheresis on co-morbidities and medications (and vice-versa) should be considered. The technical aspects of therapeutic apheresis such as a type of anticoagulant, replacement solution, vascular access, and volume of whole blood processed. Total number and/or frequency of therapeutic apheresis procedures should be addressed. The clinical and/or laboratory parameters should be established to monitor effectiveness of the treatment. The criteria for discontinuation of therapeutic apheresis should be discussed whenever appropriate. The acceptable timing of initiation of therapeutic apheresis should be considered based on clinical considerations. If the timing appropriate to the clinical condition and urgency level cannot be met, a transfer to a different facility should be considered based on the clinical status of the patient. Impact Technical issues* Therapeutic plan* Clinical and/or laboratory end-points* Timing and location the above issues should be considered and explicitly discussed in a clinical note documenting the patient history, review of systems, and physical examination. Regenerative adsorber systems consist of column pairs, which are sequentially regenerated during a treatment session, and may be reusable. If available, major publications can be found as references in individual fact sheets. Even if regulatory approval exists country-specific regulations of reimbursement for apheresis treatments as part of outpatient or in-hospital care may additionally limit the actual use. Dextran sulfate adsorption columns to remove apo-B containing lipoproteins from plasma by electrostatic interaction; 3. Direct adsorption of lipoproteins using hemoperfusion to remove apo-B containing lipoproteins from whole blood through electrostatic interactions with polyacrylate coated polyacrlyamide beads; 5. Dextran sulfate cellulose columns: same mechanism as (2) above but treats whole blood; and 6.
In another study pain medication for dogs over the counter buy aleve 250mg overnight delivery, approximately 30% of an oral dose was absorbed by Sprague-Dawley and Wistar rats pain medication for dogs natural purchase 250 mg aleve fast delivery. In rats pain research and treatment journal impact factor cheap aleve 250mg amex, slow elimination occurred and even after 5 days significant amounts were observed a better life pain treatment center golden valley az purchase aleve 250mg overnight delivery. There was no evidence of accumulation in dogs following repeated dosing for a year (Ryffel et al. Cyclosporine is a potent immunosuppressant, and is used to prevent organ and tissue rejection following transplantation. Dosing and length of time for treatment depend on the type of treatment, with transplant patients receiving higher doses than other therapeutic applications. For example in patients being treated for rheumatoid arthritis, the initial dosing is 1. For patients with kidney dysfunction or disease, the dose is much lower, starting with 2. Cyclosporine is usually given orally, but in bone marrow transplantation cyclosporine is given iv at 5-6 mg/kg daily for 3 months, followed by 12. While oral and iv administration are most common, cyclosporine formulations are also available for parenteral, rectal, ophthalmic and pulmonary aerosol administration (Ragab et al. These doses for typical treatments (at which varying levels of immunosuppression occur) provide perspective on the doses at which adverse side effects are reported. Nephrotoxicity is one of the most frequent toxic side effects observed following therapeutic dosing with cyclosporine. Cyclosporine as an immunosuppressive drug has a narrow therapeutic index (Da Silva et al. Acute nephrotoxicity caused by cyclosporine is characterized by renal vasoconstriction and renal dysfunction, and is reversible with discontinuation or reduction of the cyclosporine dose. Chronic nephrotoxicity, on the other hand, is irreversible and involves serious structure damage such as arteriolopathy and tubulointerstitial fibrosis (Lee, 2010). Kidney transplant patients have a particularly high risk of chronic kidney toxicity following long term treatment with cyclosporine (Lee, 2010; Tedesco and Haragsim, 2012). Several of these effects are likely associated with impaired kidney function, and these effects in general are consistent with the animal observations described below. Although it is a primary reference, it provides much less detail than is typically standard for such studies. Adverse effects noted include hyperventilation, drowsiness, muscular spasms, weight loss and diarrhea. Administration of 20 or 40 mg/kg-day cyclosporine to an unspecified strain of rats in the diet for an unspecified period resulted in damage to the proximal nephron and proximal tubule. In both of these studies, the highest dose was reduced (to 48 and 45 mg/kg-day, respectively) due to serious toxic effects, including mortality. At the two highest doses, adverse effects in both species included degenerative changes in the kidney and liver, changes in serum chemistry consistent with the liver and kidney effects, decreases in red blood cell markers but not white blood cells, marked neurological effects (sedation, ataxia), and atrophy of lymphoid tissue (Ryffel et al. These studies evaluated body weight, food consumption, hematology and blood chemistry, and organ weight and histopathology. In rats, the two higher doses caused atrophy of lymphoid tissues and clear nephro- and hepatotoxicity. In monkeys, cyclosporine was well tolerated with minimal toxicity, and so the high dose was increased at 4 weeks to 300 mg/kg-day. Beagle dogs (4 males/4 females per group) were administered cyclosporine by gavage in olive oil at 0, 5, 15, or 45 mg/kg-day for one year (Ryffel et al. Reversible hypertrophic gingivitis with mononuclear cell infiltration and atypical cutaneous papillomatosis occurred at 45 mg/kg-day. Other effects, including anemia, leucopenia and thrombocytosis, were attributed to malnutrition or stress. This study demonstrates a unique toxic syndrome in rabbits that is characterized by weight loss, reduced food and water consumption, and reduced movement. Dose dependent mortality was observed within 60 days of treatment, and animals had distended stomachs and intestines (Gratwohl et al.
250mg aleve mastercard. Rehabilitate Your Knee After Total Knee Replacement Surgery.