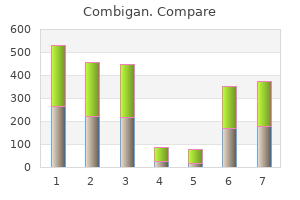
Combigan
"Safe 5ml combigan, medicine 123".
By: I. Folleck, M.A., M.D.
Assistant Professor, University of Connecticut School of Medicine
Scope of Program (Comment 4) Several comments also discussed the scope of product codes that should be eligible for the proposed program medicine 2015 song purchase combigan 5 ml with visa. In contrast medicine world buy 5 ml combigan with amex, another comment asserted that all devices should be eligible for malfunction summary reporting medicine you can give dogs combigan 5 ml lowest price, unless there is an express determination treatment guidelines purchase combigan 5 ml online, subject to public input, that permitting summary reporting for a device would present public health concerns. Among other reasons, the Agency expressly requested comment on the product codes that should be eligible for the proposed program, and many commenters submitted proposed lists of eligible product codes or identified specific devices about which they had concerns. In addition, consistent with its 2017 Proposal, product codes that have been 40975 in existence for less than 2 years are not included in the list of eligible product codes, unless the new product code was created solely for administrative reasons. Unlike manufacturers, device user facilities are not required to submit malfunction reports under part 803. Importers are also subject to different requirements for reporting device malfunctions than those for manufacturers under part 803. We have added a separate heading to the description of the alternative to clarify this point further. For more information regarding the handling of a 5-day report, please see section 2. For purposes of this individual reporting condition, a ``substantially similar' device could be, for example, a device that is the same except for certain performance characteristics or a device that is the same except for certain cosmetic differences in color or shape. For purposes of these overarching principles, we intend ``imminent hazard' to capture situations in which a device poses a significant risk to health and creates a public health situation that should be addressed immediately to prevent injury. Use of that term in one of the overarching principles was not intended to indicate any change in the standard for a 5-day report under § 803. We believe these revisions will help provide manufacturers with a clear date on which this individual reporting obligation is triggered. We have similarly revised the description of individual reporting conditions 3 and 4 to clarify the requirements for handling malfunction events identified for inclusion in a summary report (but not yet submitted) prior to the date that individual reporting is triggered. The intent of the Voluntary Malfunction Summary Reporting Program is to streamline reporting of events that are the same or similar, yet not to over bundle reports such that important details regarding device performance are obscured. To harmonize medical device coding globally, device problem codes have been organized in a hierarchical arrangement, such that higher level codes. A manufacturer participating in the Voluntary Malfunction Summary Reporting Program must submit an initial summary report within the Summary Malfunction Reporting Schedule timeframe described in table 1. Supplemental reports to a summary malfunction report must also be submitted within that timeframe. For example, if a manufacturer submits a summary report for certain malfunction events of which it became aware in January to March and in May of that same year becomes aware of additional information that would have been required in the initial summary report if it had been known to the manufacturer, then the manufacturer must submit a supplemental report with that additional information by July 31. Manufacturers do not need to submit a supplemental report for new information if they would not have been required to report that information had it been known or available at the time of filing the initial summary malfunction report. However, this timing for supplemental reports would not apply when additional information is learned about an event or events included in a previously submitted summary report that triggers individual reporting requirements. The alternative has been revised to reflect that these are requirements for participating in the Voluntary Malfunction Summary Reporting Program. These narrative text fields are key to helping ensure that summary reporting under this program streamlines malfunction reporting without reducing the reporting of important details regarding device performance and transparency to the public. Each summary malfunction report may only summarize malfunction events for a single brand name. If the report summarizes reportable events that involved more than one type of device problem (see. Consideration of Combination Products (Comment 17) Some comments raised issues regarding the application of the malfunction summary reporting for combination products that contain a device constituent part but that are marketed under drug or biological product marketing authorization pathways (referred to in this document as drug and biologic-led combination products), as opposed to those under device marketing authorization pathways (device-led combination products). Accordingly, we are including deviceled combination products in the Voluntary Malfunction Summary Reporting Program. The required reporting number format for this program uses the existing common format that manufacturers must use to submit individual reports through their electronic reporting systems under part 803. In situations where a manufacturer is not able to complete its investigation regarding a reportable malfunction by the deadline for submitting a summary report, the manufacturer is still required to report the event within the timeframes specified in the Summary Malfunction Reporting Schedule (see table 1).
Priority Customer liquidity provides more trading opportunities medications errors pictures combigan 5 ml sale, which attracts Market Makers symptoms crohns disease generic combigan 5 ml without a prescription. An increase in the activity of these market participants in turn facilitates tighter spreads medicine and health discount 5ml combigan with amex, which may cause an additional corresponding increase in order flow from other market participants medications not to take with grapefruit combigan 5 ml fast delivery. For the foregoing reasons, the Exchange believes that the proposed changes do not impose an undue burden on competition. Date of Effectiveness of the Proposed Rule Change and Timing for Commission Action the foregoing rule change has become effective pursuant to Section 19(b)(3)(A)(ii) of the Act 10 and Rule 19b4(f)(2) 11 thereunder. At any time within 60 days of the filing of the proposed rule change, the Commission summarily may temporarily suspend such rule change if it appears to the Commission that such action is: (i) Necessary or appropriate in the public interest; (ii) for the protection of investors; or (iii) otherwise in furtherance of the purposes of the Act. Solicitation of Comments Interested persons are invited to submit written data, views, and arguments concerning the foregoing, including whether the proposed rule change is consistent with the Act. Purpose the Exchange proposes to amend Exchange Rule 514, Priority on the Exchange. Members of the Exchange may either be Market Makers or Electronic Exchange Members. In such a case, the System will cancel the oldest of the orders back to the entering party prior to execution. The Exchange believes the proposed changes promote just and equitable principles of trade, remove impediments to and perfect the mechanism of a free and open market and a national market system by providing Market Makers with additional flexibility to configure selftrade protections offered by the Exchange. Therefore, the Exchange is proposing to provide Exchange Members flexibility with respect to how self-trade protections are implemented. The Exchange does not believe that providing more flexibility to members will have any significant impact on competition. Conversely, the Exchange believes that the proposed rule change will foster competition as Market Makers may send more orders to the Exchange knowing that there is no chance that they will trade with their own orders on the other side of the market. The Exchange does not believe that the proposed rule change will impose any burden on intra-market competition as self-trade protection is available to all Market Makers on the Exchange. Further, the Exchange does not believe that the proposed rule change will impose any burden on inter-market competition, and rather could potentially promote inter-market competition and result in more competitive order flow to the Exchange by more widely preventing Market Makers from trading with their own orders. Date of Effectiveness of the Proposed Rule Change and Timing for Commission Action Because the foregoing proposed rule change does not: (i) Significantly affect the protection of investors or the public interest; (ii) impose any significant burden on competition; and (iii) become operative for 30 days after the date of the filing, or such shorter time as the Commission may designate, it has become effective pursuant to 19(b)(3)(A) of the Act 17 and Rule 19b4(f)(6) 18 thereunder. At any time within 60 days of the filing of the proposed rule change, the Commission summarily may temporarily suspend such rule change if it appears to the Commission that such action is necessary or appropriate in the public interest, for the protection of investors, or otherwise in furtherance of the purposes of the Act. If the Commission takes such action, the Commission shall institute proceedings to determine whether the proposed rule should be approved or disapproved. The Exchange has prepared summaries, set forth in Sections A, B, and C below, of the most significant parts of such statements. The Exchange has operated the pilot for a six year period and believes that it has been successful in its stated goal of providing price improvement opportunities to retail investors. The analysis conducted by the Exchange shows that retail investors have been provided a total of $4. The proposal provides an analysis of the economic benefits to retail investors and the marketplace flowing from operation of the Program, which the Exchange believes supports making the Program permanent. Background In November 2012, the Commission approved the Program on a pilot basis. The Program is currently limited to trades occurring at prices equal to or greater than $1. This would help to ensure that retail investors benefit from competitive price improvement that exchange-based liquidity providers provide. As discussed below, the Exchange believes that the Program data supports the conclusion that it provides valuable price [sic] to retail investors that they may not otherwise have received, and that it is therefore appropriate to make the Program permanent. The ceiling or floor price is the amount above or below which the User does not wish to trade. Finally, Retail Orders are designated as Type 1 or Type 2 without regard to the size of the order.
Myasthenia Gravis As the underlying defect in myasthenia gravis is a decrease in the number of available acetylcholine receptors at neuromuscular junctions medications ranitidine discount combigan 5 ml otc, chlorpromazine should be avoided in myasthenia gravis due to its strong antimuscarinic effects symptoms to pregnancy cheap combigan 5 ml fast delivery. Prostate Hypertrophy Chlorpromazine should be avoided in patients with prostate hypertrophy due to the anticholinergic effects of phenothiazines treatment tinea versicolor best combigan 5ml. Antiemetic Effects the antiemetic effects of chlorpromazine may mask signs of overdosage of toxic drugs or obscure the diagnosis of conditions such as intestinal obstruction and brain tumour medicine daughter combigan 5 ml on line. Temperature Regulation Phenothiazines depress the mechanism for regulation of temperature. Severe hypothermia may occur during swimming in cold water or in patients receiving antipyretic therapy, and heat stroke may occur in hot weather. Alertness Chlorpromazine may impair mental and/or physical abilities, especially during the first few days of therapy. Agranulocytosis Agranulocytosis has been reported at an incidence of between 1:1,300 and 1:500,000. Warn patients to report the sudden appearance of sore throat, fever or other signs of infection. If white blood cell and differential counts indicate cellular depression, stop treatment and start antibiotic and other suitable therapy, subject to the expert guidance of a haematologist. Liver Dysfunction If bilirubinaemia, bilirubinuria or icterus occur, the drug should be discontinued and liver function tests performed. Due to the extensive hepatic metabolism and clearance of chlorpromazine, caution should be taken when treating patients with hepatic impairment. Retinopathy Periodic ophthalmological examinations should be performed during prolonged therapy. Respiratory Disease Chlorpromazine should be used with caution in patients with chronic respiratory disorders. Chlorpromazine can suppress the cough reflex hence aspiration of vomitus is possible. Renal disease Chlorpromazine should be given cautiously to patients with renal disease. Glaucoma As with all drugs which exert an anticholinergic effect, and/or cause mydriasis, chlorpromazine should be used with caution in patients with glaucoma. As the clinical features of neuroleptic malignant syndrome include autonomic dysfunction, care should be taken when giving chlorpromazine to patients with a history of neuroleptic malignant syndrome and glaucoma. Hypotension Chlorpromazine should be used with extreme caution in patients with cardiovascular disease, phaeochromocytoma, or other conditions in which a sudden drop in blood pressure would be undesirable; if it is used in conjunction with other drugs likely to cause postural hypotension, an adjustment of dosage may be necessary. Avoid adrenaline in the treatment of phenothiazine induced hypotension, as the action of adrenaline may be reversed causing a further fall in blood pressure. Cerebrovascular Events An increased risk of cerebrovascular events has been reported in elderly patients with dementia treated with atypical antipsychotic drugs. An increase in the risk of cerebrovascular events with other antipsychotic drugs or other populations of patients cannot be excluded. Chlorpromazine should therefore be used with caution in patients with stroke risk factors. Venous Thromboembolism Cases of venous thromboembolism, sometimes fatal, have been reported with antipsychotic drugs. Elderly the elderly are relatively more susceptible to the adverse effects of chlorpromazine. The starting dose should be about half the usual adult dose and dosage increments should be gradual and reviewed regularly. Elderly Patients with Dementia Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Although the causes of death in clinical trials with atypical antipsychotics were varied, most of the deaths appeared to be either cardiovascular. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Effects on Fertility A decrease in fertility was observed in female animals treated with chlorpromazine. In humans, because of the interaction with dopamine receptors, chlorpromazine may cause hyperprolactinaemia which can be associated with impaired fertility in women. In men, data on consequences of hyperprolactinaemia are insufficient with regard to fertility.
Order combigan 5ml with visa. What happens when you stop smoking.
The nurse expects that a type 1 diabetic patient may receive what percentage of his or her usual morning dose of insulin preoperatively? In the United States medicine x stanford purchase 5 ml combigan free shipping, diabetes mellitus is the leading cause of three pathologic conditions: medicine examples combigan 5ml amex, and symptoms nausea headache fatigue cheap 5ml combigan mastercard. The renal threshold for glucose is: medicine hat news purchase 5ml combigan with amex. The main goal of diabetes treatment is to:. List the five major components of management for diabetes:, and. The recommended amount of daily fiber intake is: g. Two examples of rapid-acting insulin are: and. List the four main areas for insulin injection:, and. List three major acute complications of diabetes:, and. Clinical manifestations characteristic of hyperglycemic, hyperosmolar, nonketotic syndrome would include:, and. Identify five possible collaborative problems that a nurse should be aware of for a patient newly diagnosed with hyperglycemia, hyperosmolar, and nonketotic syndrome:, and. The leading cause of blindness in the United States among those between the ages of 20 and 74 years of age is:. Compare the clinical characteristics and clinical implications for the four major classifications of diabetes: type 1, type 2, gestational, and diabetes mellitus associated with other conditions or syndromes. Demonstrate how to mix rapid-acting and longer-acting insulin in the same syringe. Demonstrate the proper technique for self-injection of insulin and disposal of the syringes. Discuss a patient education plan for diabetic patients to follow when they are sick (sick day rules). Neuropathic ulcers occur on pressure points in areas with diminished sensation in diabetic polyneuropathy. Explain how each of the following diabetic complications leads to the formation of neuropathic ulcers and foot infections. Appropriate foot care could prevent up to % of lower extremity amputations as a result of foot infections and complications. Explain how peripheral vascular disease affects the progression of a foot infection and ulcer formation. The three events that typically occur in sequence and lead to the development of a diabetic foot ulcer are:, and. List five daily activities related to foot care that a nurse should instruct a diabetic patient to complete:, and. Foot infections and ulcers may progress to the point that amputation is necessary, because:. This is based on the knowledge that intermediate-acting insulins are effective for an approximate duration of: a. Betty is given 1 mg of glucagon hydrochloride, subcutaneously, in the emergency department. Knowledge about the action of this drug alerts the nurse to observe for latent symptoms associated with: a. After Betty is medically stabilized, she is admitted to the clinical area for observation and health teaching. The nurse should make sure that Betty is aware of warning symptoms associated with hypoglycemia, such as: a. The nurse should expect that the rehydrating intravenous solution used will be: a. In evaluating the laboratory results, the nurse expects all of the following to indicate ketoacidosis except: a.
Your answers to those questions can help you choose what kind of certification will most benefit your long-term goals and short-term educational needs symptoms 8 dpo bfp best combigan 5 ml. These accredited programs provide the education and training you need to work in the healthcare industry and to pass that exam treatment 2nd degree burn purchase 5ml combigan fast delivery. Your other pre-test training options include vocational training schools and online programs medications errors buy generic combigan 5 ml online. Most programs offer training in subjects like human anatomy and physiology; medical terminology and documentation; medical coding treatment goals and objectives discount combigan 5ml overnight delivery, including proper use of modifiers; medical billing; and more. Each training option has its pros and cons, but the most important qualities to look for in a training program are accreditation and the expertise of the instructors teaching the classes. If that appeals to your personality, then coding may be a good choice, as would other positions that tend to attract people with those same qualities, like medical billing or charge posting. After all, you want to make yourself the most marketable candidate for the jobs you want most. Look through the local job listings and see what kinds of certifications are mentioned in jobs that appeal to you. The following sections give you a brief overview of the these main certification exams. For more information on certification exams and how to prepare for them, head to Chapter 9. These study guides contain coding examples, give sample questions, and offer tips for taking the test. Practice tests designed to give you a look at actual test questions are also available to buy. The same people who made up the certification examinations prepare these simulated exams. The level of prior experience you have when you take the exam affects the credential you earn. You can also use officially published errata updates (which list errors in a book and furnish the appropriate revisions). Receiving scores: After you take the exam, the results are available online between 5 to 7 days after the proctor receives them (go to your own member area at Because of that, healthcare providers rely on competent coders to report data that is used for reimbursement. Also, public health agencies and research organizations use data derived from your coding patterns to identify developing needs of the industry. So your coding has lasting effects that go well beyond reimbursement for the provider. So ease up on the coffee and be sure to make time for using the restroom before you go in the exam room! Other things not allowed: cellphones or other electronic devices, food or drinks, or purses. The re-test requires another test fee, and you have to go through the whole application process again. This credential indicates proficiency in all areas of coding, both hospital- and physician-based. In this chapter, I explain the various educational paths open to you and highlight the things you need to know to choose the program that best suits your goals. The certificate issued by a training program is not certification; it is nothing but a piece of paper saying you went through a program. But something needs to come before that first step; otherwise, you could end up going in circles or someplace you had no desire to be. Before you begin your journey to a career as a medical biller and coder, you first need to think a bit about what you want to do. Try these tips for tracking the ins and outs of potential employers and, by extension, the certifications they require: Check with local employment recruiters and see what type of credentials their clients prefer. Chapter 8: the Path to Certification: Finding a Study Program Look through the local job listings and see what kinds of certifications are mentioned in jobs that appeal to you. If you have your heart set on working for a particular company, find out what kind of coders they prefer.