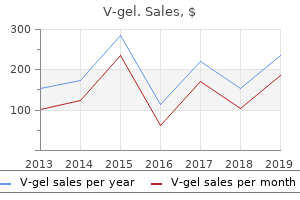
V-gel
"Discount v-gel 30 gm on line, herbals hills".
By: R. Merdarion, M.B. B.CH., M.B.B.Ch., Ph.D.
Associate Professor, New York Institute of Technology College of Osteopathic Medicine at Arkansas State University
Further biochemical profiles may be necessary to identify a block in testosterone biosynthesis wtf herbals cheap 30 gm v-gel overnight delivery, decreased 5-alpha-reductase activity or androgen insensitivity (3) herbals meds generic 30gm v-gel fast delivery. Gonadal inspection and biopsy are necessary and can be done laparoscopically in many cases by an experienced pediatric urologist or pediatric surgeon rm herbals buy 30gm v-gel with amex. This will not be necessary in established cases of congenital adrenal hyperplasia and Turner syndrome herbs books v-gel 30gm low price. The classification of intersex disorders are most conveniently divided into four main groups based on gonadal histology: 1) Female pseudohermaphrodites (two normal ovaries present). This virilization of the female fetus is secondary to androgens from either the maternal circulation or the fetal adrenal gland. Salt wasting occurs in 75 percent of patients with classical disease, and is evident within the first two weeks of life, with resultant hyponatremia, hypokalemia, and inappropriate sodium wasting (high urine sodium despite hyponatremia) due to low serum aldosterone and elevated plasma renin activity (7). It is crucial to recognize this potentially life-threatening condition in the newborn period and institute replacement of cortisol and mineralocorticoid as necessary. Other causes of female pseudohermaphrodism are maternal progesterone ingestion (with androgenic side effects) administered during pregnancy to prevent abortion, a virilizing ovarian or adrenal tumor in the mother, or idiopathic causes. Cellular testosterone sensitivity is abnormal in 80 percent of cases, and testosterone production is deficient in the remaining 20 percent. The causes include androgen insensitivity, gonadotropic failure, Leydig cell agenesis, bilateral vanishing testes syndrome, persistent mullerian duct syndrome, testosterone biosynthesis defects and 5-alphareductase deficiency (5). Patients may have abnormal male genitalia, ambiguous genitalia, or female genitalia with palpable or nonpalpable testes, depending on the completeness and nature of the defect and the extent of gonadotropin oversecretion. Androgen insensitivity is the most common (1 in 20,000 male births) cause of male pseudohermaphrodism and results from dysfunction or reduction of the androgen receptor. For complete testicular feminization, the androgen receptor is absent or completely nonfunctional. The pituitary and hypothalamus are insensitive to testosterone and thus secrete large amounts of gonadotropins, which results in the oversecretion of testosterone and estrogen (5). Breast development, general body habitus, and distribution of body fat are female in character. The clitoris is normal or small, and the vagina is short with a blind ending, but the external genitalia are female in appearance. All internal genitalia are absent (no uterus or ovaries) except for the gonads, which have the histologic appearance of undescended testes (6). Because of increased tumor risk in the undescended testes (5% to 10%), gonadectomy is recommended after puberty. Patients with complete androgen insensitivity syndromes (testicular feminization) are normal phenotypic females who present during childhood with one or both testes palpable in an inguinal hernia, or with amenorrhea at puberty. A few are diagnosed based on discrepancy between prenatal karyotype and phenotype at birth. The diagnosis is based on clinical and family history, endocrine studies and, if indicated, androgen binding analysis in genital skin fibroblasts (5). The diagnosis is often made at puberty, when progressive virilization associated with penile growth, attainment of male secondary sex characteristics, testicular descent, and a change in gender identity may occur (5). The internal male genitalia are normal, and the testes are located in the labioscrotal pouch. The external genitalia typically show severe perineoscrotal hypospadias and a blind vaginal pouch opening into the urogenital sinus or urethra. At puberty, normal levels of luteinizing hormone and testosterone result in masculinization of the external genitalia, and breasts do not develop (5,6). True hermaphrodism is a rare condition in which ovarian and testicular tissue exist in the same individual. Patients most commonly have ambiguous genitalia, but near-normal female and male genitalia may be present. A unicornuate or bicornuate uterus is usually present, and the differentiation of the genital ducts is determined by the ipsilateral gonad, with the ovary usually located on the left side (5).
At present rumi herbals cheap 30gm v-gel with visa, this reaction is known to occur with cimetidine xena herbals buy v-gel 30gm with amex, ketoconazole himalaya herbals review generic v-gel 30 gm with visa, fluvoxamine humboldt herbals v-gel 30gm sale, fluoxetine, and omeprazole. The data currently available are inadequate to determine the mutagenic potential of diazepam. Pediatric Use Safety and effectiveness in pediatric patients below the age of 6 months have not been established. Geriatric Use In elderly patients, it is recommended that the dosage be limited to the smallest effective amount to preclude the development of ataxia or oversedation (2 mg to 2. Extensive accumulation of diazepam and its major metabolite, desmethyldiazepam, has been noted following chronic administration of diazepam in healthy elderly male subjects. Metabolites of this drug are known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Hepatic Insufficiency Decreases in clearance and protein binding, and increases in volume of distribution and half-life have been reported in patients with cirrhosis. Delayed elimination has also been reported for the active metabolite desmethyldiazepam. The following have also been reported: Central Nervous System: confusion, depression, dysarthria, headache, slurred speech, tremor, vertigo Gastrointestinal System: constipation, nausea, gastrointestinal disturbances Special Senses: blurred vision, diplopia, dizziness Cardiovascular System: hypotension Psychiatric and Paradoxical Reactions: stimulation, restlessness, acute hyperexcited states, anxiety, agitation, aggressiveness, irritability, rage, hallucinations, psychoses, delusions, increased muscle spasticity, insomnia, sleep disturbances, and nightmares. Inappropriate behavior and other adverse behavioral effects have been reported when using benzodiazepines. Urogenital System: incontinence, changes in libido, urinary retention Skin and Appendages: skin reactions Laboratories: elevated transaminases and alkaline phosphatase Other: changes in salivation, including dry mouth, hypersalivation Antegrade amnesia may occur using therapeutic dosages, the risk increasing at higher dosages. Because of isolated reports of neutropenia and jaundice, periodic blood counts and liver function tests are advisable during long-term therapy. Postmarketing Experience: Injury, Poisoning and Procedural Complications: There have been reports of falls and fractures in benzodiazepine users. The risk is increased in those taking concomitant sedatives (including alcohol), and in the elderly. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving diazepam or other psychotropic agents because of the predisposition of such patients to habituation and dependence. Once physical dependence to benzodiazepines has developed, termination of treatment will be accompanied by withdrawal symptoms. Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol have occurred following abrupt discontinuance of diazepam. These withdrawal symptoms may consist of tremor, abdominal and muscle cramps, vomiting, sweating, headache, muscle pain, extreme anxiety, tension, restlessness, confusion and irritability. In severe cases, the following symptoms may occur: derealization, depersonalization, hyperacusis, numbness and tingling of the extremities, hypersensitivity to light, noise and physical contact, hallucinations or epileptic seizures. The more severe withdrawal symptoms have usually been limited to those patients who had received excessive doses over an extended period of time. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed. Chronic use (even at therapeutic doses) may lead to the development of physical dependence: discontinuation of the therapy may result in withdrawal or rebound phenomena. Rebound Anxiety: A transient syndrome whereby the symptoms that led to treatment with Valium recur in an enhanced form. It may be accompanied by other reactions including mood changes, anxiety, and restlessness. Since the risk of withdrawal phenomena and rebound phenomena is greater after abrupt discontinuation of treatment, it is recommended that the dosage be decreased gradually. In more serious cases, symptoms may include ataxia, diminished reflexes, hypotonia, hypotension, respiratory depression, coma (rarely), and death (very rarely). Gastric lavage should be undertaken with the airway protected if the patient is unconscious.
Often the approach does not even distinguish whether it is a brand name product or a generic product that is being used herbals incense cheap 30 gm v-gel overnight delivery. Even when the distinction is made zen herbals order v-gel 30 gm fast delivery, for example most Medicaid datasets use the National Drug Code to identify specifically the drug himalaya herbals products buy v-gel 30 gm low price, the manufacturer herbals interaction with antihistamines buy generic v-gel 30 gm on-line, the dosage form, and the dose, one is inevitably left with questions about whether a brand name is being billed for, while a generic drug is dispensed. In addition, such studies raise concerns about how to define the clinical outcome variable. The studies described above used outcomes like number of physician visits, number of hospitalizations, and use of adjunctive therapy to obtain an estimate of drug efficacy. Using these outcomes, the investigators first analyzed the baseline data, comparing the experience, prior to switching, of those who ultimately switched to generic products to the experience of those who did not later switch to a generic product. In each of the three studies, the future switchers were different from the future nonswitchers, prior to the switch. Thus, it appears that patients who were to be switched to generic products were different than patients who stayed on the brand name products: confounding by indication was indeed operating. Parenthetically, because of this, and questions about the uncertain interpretability of the clinical outcomes, it was elected not to publish the results of these papers. For example, one could perform a cohort study comparing treated patients to untreated patients, and determine whether the clinical outcomes they experience and the cost of the medical care they subsequently receive is different. In such a study, one would need to consider the possibility of confounding by the indication for both the clinical outcome and the cost variables. It should be noted that the indication may have different effects on the clinical outcomes and the costs. Thus, while performing the clinical outcome assessment, one needs to consider and, potentially, quantify the implications of the indication for the treatment on the clinical outcome variable. In contrast, while performing the cost assessment, one needs to consider and, potentially, quantify the cost implications of the indication on both the clinical outcomes and the costs. The subject of health economics as applied to drug use is discussed in more detail in Chapter 35. Vaccines In the last several years, nonexperimental study designs have been widely used to evaluate the efficacy of vaccines. However, the relative infrequency of the diseases the above vaccines are designed to prevent, particularly in populations which are partly vaccinated, make use of this design difficult, although not impossible. However, for most vaccines, an individual physician is not likely to give only some of his eligible patients the vaccine, withholding it from other eligible patients. Nonexperimental studies of such questions should produce valid results, therefore. We refer the interested reader to some methodologic papers on the subtleties of designing nonexperimental studies of vaccine efficacy. Although this does not directly relate to drugs, the methodological implications are the same, and have been better enunciated than in the pharmacoepidemiology literature. The use of nonexperimental study designs to evaluate the efficacy of cancer screening programs will be briefly discussed here, therefore. Once again, ideally questions about the value of screening would be addressed using randomized clinical trials. Only a very small fraction of those in a broad screening program could be expected to benefit from the screening program. Thus, randomized clinical trials of screening can be expensive and may require years to complete. Again, they raise similar methodologic considerations of confounding by indication.
The need to evaluate data from all trials herbals meds purchase v-gel 30gm fast delivery, with different kinds of patient population herbs near me buy v-gel 30gm on line, differing designs rumi herbals chennai 30gm v-gel with visa, and differing durations makes epidemiologic approaches especially appropriate qarshi herbals generic v-gel 30 gm fast delivery. The approach required is one that recognizes the limited nature of the data and avoids overinterpretation in either direction. Investigators are asked to provide a clinical assessment as to the causality of each event. Such assessments are a necessary part of safety monitoring in clinical research, although they are known to be subjective and imprecise14 (see also Chapter 32). However, for serious, uncommon adverse events where the clinical features of the drug related cases could be similar to those of non-drug-related cases, it can sometimes be helpful to supplement clinical causality assessments of individual cases by epidemiologic the guideline also emphasizes the difference between the formal, prespecified efficacy evaluation and the more exploratory approaches used in safety evaluation in pre-approval clinical trials: Approaches useful for evaluating the safety of a drug under development generally differ substantially from those useful in evaluating its effectiveness. In designing these trials, critical efficacy endpoints are identified in advance and sample sizes are estimated to permit an adequate test of the null hypothesis. In fact, the safety endpoints are generally not known prior to the conduct of these trials, and for many of the observed safety outcomes, one can assume that the available studies are underpowered. The epidemiologic literature provides several sets of criteria for helping to decide whether an empirical association is likely to be causal. The best known criteria are those proposed in 1965 by Bradford-Hill to help evaluate evidence linking cigarette smoking with lung cancer16 (see also Chapter 2). The nine Bradford-Hill criteria are discussed briefly below as they relate to evaluation of adverse experiences in premarketing clinical trials. It is also important to recognize that the absence of any association between a drug and any given adverse event has to be judged in the context of the limited amount of patient exposure in pre-approval clinical trials. The safety evaluation during clinical drug development is not expected to characterize rare adverse events, for example, those occurring in less than 1 in 1000 patients. Strength of Association this is commonly quantified in terms of a suitably adjusted hazard function ratio or risk ratio (relative risk), rather than a p-value. In general, the farther the ratio is from unity, the less likely it could be entirely attributable to imbalances in risk factors between groups. An exception to this occurs when the ratio is based on very small numbers or highly influenced by only a few cases. In addition, it is worth noting that with the large number of different kinds of adverse events often seen in large clinical trials, it is quite likely that some risk ratios far from unity will occur by chance alone. Multiple comparisons not only distort p-values, but can also bias risk ratios and their confidence intervals away from unity. This happens because screening many different adverse event terms and selecting those with very low pvalues inherently selects for relative risks that are biased away from unity. This type of selection bias, a form of regression to the mean, is common to all programs of screening for unusually large or small values and is well known in the statistics literature. One example where this appears to have occurred is in some observational studies of vasectomy and prostate cancer. Consistency the original wording was, ``Has [the association] been repeatedly observed by different persons, in different places, circumstances and times. Results that show a consistently elevated incidence on drug across studies are generally more convincing than those in which the elevated risk is largely due to one study. This study was the only outpatient study and thus these were the only patients who had an opportunity to have the event by virtue of having sun exposure. Especially in the evaluation of adverse experiences from studies without a comparison group, it sometimes occurs that early symptoms of a disease which is present but not yet recognized lead a patient to be prescribed a drug, which then appears to be the cause of the disease when it is eventually diagnosed. This has been called ``protopathic bias' and is a special case of the broader concept of ``confounding by indication' (see also Chapter 34). A classic example of this form of bias was seen in uncontrolled postmarketing surveillance studies of cimetidine, where a higher than expected incidence of gastric carcinoma was found among users than among nonusers. It is likely that many of the cancers were present but undiagnosed at the time the cimetidine was started. Subsequent studies showed that elevations in gastric cancer risk diminished with duration of followup, returning to baseline with long term use. Thus, an elevated incidence of cancer in patients who had been taking a drug for several years would be of more concern than would an elevated incidence in the first year of therapy.
Order v-gel 30 gm on line. My Skincare routine using Himalaya 💪🏽.