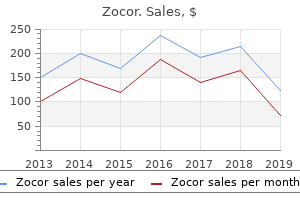
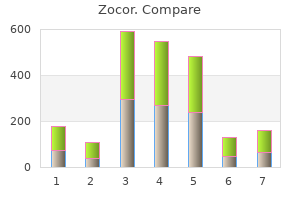
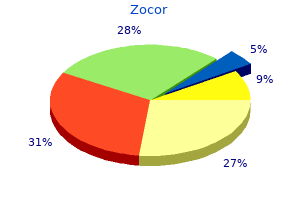
Zocor
"Purchase zocor 10mg with visa, cholesterol ratio greater than 6".
By: F. Hamid, M.B.A., M.B.B.S., M.H.S.
Deputy Director, Medical College of Georgia at Augusta University
They reduce the cholesterol level in blood and are antiatherogenic (see Chapter 25) cholesterol medication bad quality zocor 5mg. Whole cereals cholesterol chart mmol buy zocor 5 mg overnight delivery, pulses high cholesterol foods diet cheap zocor 10 mg with visa, leafy vegetables and fruits contain good quantity of fiber cholesterol precursor generic 5mg zocor overnight delivery. Diet rich in fiber improves bowel motility, prevents constipation, decreases reabsorption of bile acids thus lowering cholesterol level and improves glucose tolerance. For hypoglycemic and hypolipidemic effect, see glycemic index at the end of this Chapter. The beneficial effect is more with soluble fiber present in vegetables and only a diet having plenty of vegetables and green leaves will have the desired effect. The inclusion of fiber rich food in weight reducing diets is found to be helpful, since it provides a feeling of fullness without consumption of excess calories. In developed countries, the percentage of calories derived from fats may be as high as 40%, but in the developing countries it is much less, around 10%. A minimum intake of lipids is essential since the requirements of fat soluble vitamins and essential fatty acids are to be met. Fatty acids in oils Fat or oil Saturated (%) 75 9 86 18 Mono unsa- Poly turated unsatu(%) rated (%) 20 12 19 12 46 5 79 65 2 36 Butter/ghee (*) Safflower oil Coconut oil (*) Ground nut oil iii. When enough carbohydrates are present in the diet, the amino acids are not used for yielding energy. For the synthesis of body proteins, all the essential amino acids should be supplied in adequate quantities at the same time. Cysteine and tyrosine can be synthesized, when methionine and phenyl alanine are available; thus requirement of the precursor amino acid is determined by the availability of the product. The remaining amino acids can be synthesized, provided there is enough supply of proteins in total. Only 3 amino acids (alanine, aspartate and glutamate) are truly dispensable, as they can be synthesized from pyruvate, oxaloacetate and alpha ketoglutarate respectively; and these precursors are generally available in plenty. Cotton seed oil 26 (*) Butter/ghee contains short chain fatty acids and coconut oil contains medium chain fatty acids (see Chapter 8). Recommended protein allowances Infants Children up to 10 years Adolescent boys girls 2. It is widely used in food industry, since it increases the shelf life of fried food. Trans fatty acids adversely affect endothelial function and aggravate insulin resistance and diabetes. It is high in processed foods and bakery products, where hydrogenated vegetable oils are used for cooking. Nitrogen balance can be actually measured by calculating the dietary intake of protein nitrogen (16% of the weight of protein) and measuring the daily excretion. This is applicable in the case of infants, children, adolescents, pregnancy, lactation and convalescence. Growth: During the period of active growth, a state of positive nitrogen balance exists. On an average when a person gains 5 kg, about 1 kg proteins are added to the body. For this, about 160 g of nitrogen has to be retained, so he/she has to be in positive nitrogen balance. Hormones: Growth hormone, insulin and androgens promote positive nitrogen balance, while corticosteroids cause a negative nitrogen balance. Pregnancy: A pregnant woman will be in a state of positive nitrogen balance due to the growth of fetus. Convalescence: A person convalescing after an illness or surgery will be in positive nitrogen balance, due to active regeneration of tissues. Acute illness: Negative nitrogen balance is seen in subjects immediately after surgery, trauma and burns.
H H H5C2 H C2H5 = H5C2 C2H5 H H H5C2 C2H5 = H H5C2 H trans-1 how many cholesterol in shrimp buy zocor 20 mg on line,2-Diethylcyclopentane H C2H5 cis-1 cholesterol levels new guidelines discount zocor 5mg with visa,2-Diethylcyclopentane Physical properties of cycloalkenes Cycloalkenes are nonpolar molecules like alkanes cholesterol levels for statins 20 mg zocor sale. As a result how to remove cholesterol in eggs 5mg zocor with mastercard, they tend to have low melting and boiling points compared with other functional groups. Usually alkanes are obtained through refinement or hydrogenation of petroleum and coal. Compounds containing five-membered rings (cyclopentane) and sixmembered rings (cyclohexane) are especially common. H2O Selective reduction of aldehydes or ketones, either by Clemmensen reduction (see Section 5. In fact, it is often convenient to regard the hydrocarbon framework of a molecule as an unreactive support for the more reactive functional groups. Combustion or oxidation of alkanes Alkanes undergo combustion reaction with oxygen at high temperatures to produce carbon dioxide and water. Oxidation of saturated hydrocarbons is the basis for their use as energy sources for heat. The two smaller cycloalkanes react with hydrogen, even though they are not alkenes. In the presence of a nickel catalyst, the rings open up, and form corresponding acyclic (open chain) alkanes. H H C H Chloromethane Methyl chloride H Cl H3C C H Chloroethane Ethyl chloride Cl А Based on the number of alkyl groups attached to the CА X unit, alkyl halides are classed as primary (1), secondary (2) or tertiary (3). Physical properties of alkyl halides Alkyl halides have considerably higher melting and boiling points compared with analogous alkanes. The boiling points also increase with increasing atomic weight of the halogen atom. Thus, alkyl fluoride has the lowest boiling point and alkyl iodide has the highest boiling point. Alkyl halides are insoluble in water as they are unable to form hydrogen bonds, but are soluble in nonpolar solvents. The CА X bonds in alkyl halides are highly polar due to the higher electronegativity and polarizability of the halogen atoms. Halogens (Cl, Br and I) are good leaving groups in the nucleophilic substitution reactions. The electronegativity of halides decreases and the polarizability increases in the order of: F > Cl > Br > I. H H Chloromethane µ - + H C Cl Reactions of alkyl halides Alkyl halides undergo not only nucleophilic substitution but also elimination, and both reactions are carried out in basic reagents. Often substitution and elimination reactions occur in competition with each other. In general, most nucleophiles can also act as bases, therefore the preference for elimination or substitution is determined by the reaction conditions and the alkyl halide used. H3C - Mg + H3C - Li + Organometallic compounds When a compound has a covalent bond between a carbon and a metal, it is called an organometallic compound. The most common types of organometallic compound are Grignard reagents, organolithium reagents and Gilman reagents (lithium organocuprates, R2CuLi). A carbonmetal bond is polarized, with significant negative charge on the carbon, because metals are electropositive. Organometallic reagents react readily with hydrogen atoms attached to oxygen, nitrogen or sulphur, in addition to other acidic hydrogen atoms. They react with terminal alkynes to form alkynides (alkynyl Grignard reagent and alkynyllithium) by acidbase reactions (see Section 4. Alkynides are useful nucleophiles for the synthesis of a variety of other compounds (see Section 4. Organolithium reagents react similarly to Grignard reagents, but they are more reactive than Grignard reagents. They react readily with acid chlorides, but do not react with aldehydes, ketones, esters, amides, acid anhydrides or nitriles.
Or good bad cholesterol foods list cheap 40 mg zocor visa, "Cottrell current" could mean the amount of electric charge at a planar electrode in a diffusion controlled reaction that varies with time according to the Cottrell equation high cholesterol foods chart buy generic zocor 5 mg on-line. But cholesterol hdl ratio canada order zocor 20 mg without prescription, the parties argue that the Court must make the definition of Cottrell current more specific with respect to how closely the current must follow the Cottrell equation cholesterol in liquid eggs order zocor 10mg on-line. Starting with the claim language, all of the independent claims of the 268 patent refer to measurement of the "Cottrell current" after the oxidation reaction has been "substantially completed" or has gone "substantially to completion. That current then either "determines," "is correlated to ," or "is proportional to" the amount of biologically significant compound in the sample. Two of the claims refer to the concept that the Cottrell current is proportional to the concentration of the biologically significant compound. However, other claims suggest that the relationship between Cottrell current and concentration is not "proportional to" but "correlates with. Similarly, Claim 33 requires "correlating the measured Cottrell current to the amount of cholesterol in the blood sample. The patent states that in this method of quantification, the measurement of a diffusion controlled current at an accurately specified time. Measurements by the method according to the present invention of the current due to the reoxidation of the acceptors were found to be proportional to the glucose concentration in the sample. The equation set out in this portion of the patent is the Cottrell equation for measuring the current in a diffusion solution using a single planar electrode. Moreover, this description identifies one of the parameters for the Cottrell equation: a diffusion controlled reaction. This requirement is likely well known to one skilled in the art because a basic electrochemistry text teaches that "the important concept in [chronoamperometry] is diffusion-controlled oxidation or reduction. Furthermore, according to the specification, Figure 8 of the 268 patent "is a graphical representation of Cottrell current as a function of glucose concentration. Again, the label of the graph suggests that "Cottrell current" is current that varies in accordance with the Cottrell equation. However, the graph also shows that for a sample containing zero millimoles of glucose, there is a measurable current. Roche argues that this suggests that the invention method is less than an "ideal" one because for zero millimoles of glucose, the Cottrell equation produces a result of zero microamperes of current. This current decays rapidly when the electrode has been charged to the applied potential. This phenomenon, then, is apparently known to one skilled in the art of chronoamperometry. Therefore, Figure 8 in the specification suggests that there is some predictable variation from the Cottrell equation based on practical or experimental error in the method practiced by the 268 patent. Roche also argues that the lines representing Cottrell current in Figure 8 deviate from the Cottrell equation because they show the current reaching a plateau short of zero for each glucose concentration. Bard testified that such plateaus could be consistent with results obtained because of convection (movement of the sample fluid by external means rather than diffusion) or because of a non-planar electrode. It states, in relevant part: the Cottrell equation states that the product it<1/2> should be a constant K for a diffusion-controlled reaction at a planar electrode. Deviation from this constancy can be caused by a number of situations, including nonplanar diffusion, convection in the cell, slow charging of the electrode during the potential step, and coupled chemical reactions. But, again, it seems these responses are predictable and do not change the fundamental decay of the current with respect to time. Therefore, the patent specification does teach, to one skilled in the art, some predictable variation from the Cottrell equation parameters. The Court must use the clues in the 268 patent claims, specification and prosecution history to clarify which Cottrell equation parameters are necessary for "Cottrell current. When a reducing potential is imposed instantaneously on a stationary working electrode in quiescent solution, current will rise sharply and then decay as the electroactive species in the electrode vicinity is depleted by electrolysis. The magnitude of the - 693 - Jump to: A B C D E F G H I J K L M N O P Q R ST UVW XY Z current is proportional to the bulk concentration of electroactive species and is related to the electrode potential through the Nernst equation, Eq. Thus, under these conditions the current is diffusion-controlled and decays with 1/t<1/2>.
Hypersensitivity is the over-reaction of the immune system high cholesterol foods to eat generic 40mg zocor overnight delivery, often resulting in unwanted tissue destruction cholesterol medication day or night buy zocor 20 mg lowest price. This is responsible for caseation and lung cavity formation in the case of tuberculosis cholesterol levels malaysia 20 mg zocor overnight delivery, granulomatous skin lesions in tuberculoid leprosy cholesterol food amounts cheap 5 mg zocor with visa, skin lesions in herpes simplex and contact hypersensitivity to chemicals and plant products. Effector Mechanisms the following are the immunological effector mechanisms by which foreign cells are destroyed or particles are removed: 1. Immunity against infections: T cells mediate effective immunity against bacteria such as mycobacteria, many viruses and almost all parasites. Rejection of allograft: When an organ (heart, kidney) is transplanted from one person to another, it is called allograft. Body tries to reject such transplanted organs, mainly by T cell mediated mechanism. Tumor cell destruction: Although other mechanisms are also involved in killing tumor cells, T cell activity is the predominant one. They are necessary for optimal antibody production by plasma cells and for generation 2. The antigen-antibody reaction leads to activation of complement system, which destroys the foreign cells. The antibodies can destroy the target cells by the following mechanisms: (1) Classical complement pathway. Only very small amounts of antibody are required; so this mechanism is effective in areas where antibody concentration may be minimal. Macrophages Phagocytosis is the nonspecific mechanism by which body tries to eliminate invading organisms. Foreign materials are ingested by the phagocytes and later digested intracellularly. The myeloperoxidase present inside the phagocytes destroys the bacteria (Chapter 20). When a foreign particle enters the body, the macrophages phagocytose it, and present the antigens to the lymphocytes. Most of the immunoglobulins have the gamma mobility; but some may move along with beta or even with alpha globulins. This property is widely used in purification of proteins and for affinity chromatography. This affinity is based on the complementary nature of the three dimensional structure of antigen and antibody. If there is a protuberance in the antigen, there is a corresponding cleft in the antibody structure. In 1962, Rodney Porter and Gerald Edelman independently proposed the structure for immunoglobulin molecule, for which both of them were awarded Nobel prize in 1972. Papain cuts the immunoglobulin molecule at a site towards the amino terminal part of the disulphide linkages. Pepsin cleaves the molecule towards the carboxy terminal part of the disulphide linkages, so that one F(ab)2 and one Fc portion are produced acids and L chains made up of 214 amino acids. Depending on the heavy chain make up, the immunoglobulins are differentiated into 5 major classes. Variable and Constant Regions Both the heavy and light chains contain relatively variable (V) and constant (C) regions with regard to their amino acid composition. The first 108 amino acids in light chains and first 118 amino acids in heavy chains constitute the variable Heavy and Light Chains the structure of lgG molecule is shown in. It is made up of 2 heavy (H) chains and 2 light (L) chains, combined through disulfide bridges. In the case of lgG, H chains are composed of 440 amino 556 Textbook of Biochemistry; Section G: Advanced Biochemistry Table 49. Characteristics of different immunoglobulin classes IgG Nomenclature of heavy chain Heavy chain domains 4 IgA 4 2 S and J 3,85,000 11 S 8-10 150-300 mg 6 5 IgM 5 5 J piece 9,70,000 19 S 12 50-200 mg 10 95 IgD 4 1 - 1,85,000 7S 10-13 1-10 mg 3 75 IgE 5 1 - 1,90,000 8S 11-12 1. Here the amino acid sequence can vary in H and L chains, so that the body could synthesize enormous varieties of different antibodies.
Changes in drug therapy may warrant additional viral load studies to verify efficacy of modified therapy how many cholesterol in an eggs generic 20mg zocor. Inform the patient that the test is primarily used to monitor disease progression and effectiveness of retroviral therapy cholesterol in chicken generic zocor 10mg online. Stress the importance of following the care plan for medications and followup visits list of cholesterol lowering foods zocor 10mg with mastercard. Inform the patient that subsequent requests for follow-up blood work at regular intervals should be anticipated cholesterol za niski poziom purchase 40 mg zocor mastercard. Educate the patient as to the risk of infection related to immunosuppressed inflammatory response and fatigue related to decreased energy production. Sensitivity to social and cultural issues, Counsel the patient, as appropriate, regarding risk of transmission and proper prophylaxis, and reinforce the importance of strict adherence to the treatment regimen, including consultation with a pharmacist. Refer to the Hematopoietic and Immune System tables at the end of the book for related tests by body system. Tube 1 is used for chemistry and serology testing, tube 2 is used for microbiology, tube 3 is used for cell count, and tube 4 is used for miscellaneous testing. Specimens for analysis are most frequently obtained by lumbar puncture and sometimes by ventricular or cisternal puncture. Lumbar puncture can also have therapeutic uses, including injection of drugs and anesthesia. Inform the patient that the test is primarily used to assist in the differential diagnosis of infection or hemorrhaging in the brain. It is also used in the evaluation of other conditions with significant neuromuscular effects. Inform the patient that the position required may be awkward, but that someone will assist during the procedure. Address concerns about pain and explain that a stinging sensation may be felt when the local anesthetic is injected. Instruct the patient to report any pain or other sensations that may require repositioning the spinal needle. Prepare the site-usually between L3 and L4, or between L4 and L5-with povidone-iodine and drape the area. Normal pressure for an adult in the lateral recumbent position is 90 to 180 mm H2O; normal pressure for a child age 8 years or younger is 10 to 100 mm H2O. These values depend on the body position and are different in a horizontal or sitting position. Provide information regarding vaccinepreventable diseases when indicated (encephalitis, influenza, meningococcal diseases). Refer to the Immune and Musculoskeletal System tables at the end of the book for related tests by body system. Decreased production of this globulin causes copper to be deposited in body tissues such as the brain, liver, corneas, and kidneys. Nutritional considerations: Instruct the patient with copper deficiency to increase intake of foods rich in copper, as appropriate. High intake of zinc, iron, calcium, and manganese interferes with copper absorption. Copper deficiency does not normally occur in adults; however, patients receiving long-term total parenteral nutrition should be evaluated if signs and symptoms of copper deficiency appear, such as jaundice or eye color changes. Refer to the Hepatobiliary System table at the end of the book for related tests by body system. The lungs, filled with air, are easily penetrated by x-rays and appear black on chest images. Additional projections that may be requested are obliques, lateral decubitus, or lordotic views. Chest images should be taken on full inspiration and erect when possible to minimize heart magnification and demonstrate fluid levels. Rib detail images may be taken to delineate bone pathology, useful when chest radiographs suggest fractures or metastatic lesions. Fluoroscopic studies of the chest can also be done to evaluate lung and diaphragm movement. In the beginning of the disease process of tuberculosis, asthma, and chronic obstructive pulmonary disease, the results of a chest x-ray may not correlate with the clinical status of the patient and may even be normal. Address concerns about pain and explain that no pain will be experienced during the test.
Cheap zocor 10 mg free shipping. THE TRUTH About Cholesterol and Saturated Fat - Eating Healthy with Craig Emmerich.