Renagel
"Cheap 400mg renagel with visa, symptoms of gastritis mayo clinic".
By: A. Rhobar, M.B. B.CH. B.A.O., M.B.B.Ch., Ph.D.
Clinical Director, Johns Hopkins University School of Medicine
Promoting Home- and Community-Based Care Teaching Patients Self-Care N · Direct patient education toward symptom management and monitoring for complications gastritis y embarazo buy 400 mg renagel amex. Nephrotic Syndrome Nephrotic syndrome is a primary glomerular disease characterized by proteinuria gastritis diet ����� generic renagel 800 mg otc, hypoalbuminemia gastritis zunge purchase 800 mg renagel amex, diffuse edema gastritis diet ���������� buy renagel 400mg on-line, high serum cholesterol, and hyperlipidemia. It is seen in any condition that seriously damages the glomerular capillary membrane, causing increased glomerular permeability with loss of protein in the urine. It occurs with many intrinsic renal diseases and systemic diseases that cause glomerular damage. It is not a specific glomerular disease but a constellation of clinical findings that result from the glomerular damage. It is usually soft, pitting, and commonly occurs around the eyes (periorbital), in dependent areas (sacrum, ankles, and hands), and in the abdomen (ascites). Assessment and Diagnostic Findings · Protein electrophoresis and immunoelectrophoresis to determine type of proteinuria exceeding 3. N Nephrotic Syndrome 463 · Needle biopsy of the kidney for histologic examination to confirm diagnosis. Nursing Management · In the early stages, nursing management is similar to that of acute glomerulonephritis. Medical Management A weight loss diet in conjunction with behavioral modification and exercise is usually unsuccessful. Nursing Management Nursing management focuses on care of the patient after surgery. General postoperative nursing care is similar to that for a patient recovering from a gastric resection, but with great attention given to the risks of complications associated with morbid obesity. O 466 Osteoarthritis (Degenerative Joint Disease) · For patients who undergo laparoscopic or open Roux-en-Y procedures and have one or more Jackson Pratt drains, teach the patient and family how to empty, measure, and record the amount of drainage. Clinical Manifestations · Pain, stiffness, and functional impairment are primary clinical manifestations. Assessment and Diagnostic Findings · X-ray study shows narrowing of joint space and osteophytes (spurs) at the joint margins and on the subchondral bone. O Osteoarthritis (Degenerative Joint Disease) 467 · There is a weak correlation between joint pain and synovitis. Medical Management Management focuses on slowing and treating symptoms because there is no treatment available that stops the degenerative joint disease process. Managing pain and optimizing functional ability are the major goals of nursing intervention, 468 Osteomalacia and helping patients understand their disease process and symptom pattern is critical to a plan of care. Osteomalacia Osteomalacia is a metabolic bone disease characterized by inadequate mineralization of bone. Osteomalacia may result from failed calcium absorption (malabsorption) or excessive loss of calcium (celiac disease, biliary tract obstruction, chronic pancreatitis, bowel resection) and loss of vitamin D (liver and kidney disease). Additional risk factors include severe renal insufficiency, hyperparathyroidism, prolonged use of antiseizure medication, malnutrition, and insufficient vitamin D (eg, from inadequate dietary intake or inadequate sunlight exposure). O Osteomyelitis 469 Assessment and Diagnostic Findings · X-ray studies, bone biopsy shows increased osteoid (demineralized bone matrix). Gerontologic Considerations Promote adequate intake of calcium and vitamin D and a nutritious diet in disadvantaged elderly patients. Reduce incidence of fractures with prevention, identification, and management of osteomalacia. When osteomalacia is combined with osteoporosis, the incidence of fracture increases. It may occur by extension of soft tissue infections, direct bone contamination (eg, bone surgery, gunshot wound), or hematogenous (bloodborne) 470 Osteomyelitis spread from other foci of infection. Other pathogenic organisms frequently found include Gram-positive organisms that include streptococci and enterococci, followed by Gram-negative bacteria that include pseudomonas species. Patients at risk include poorly nourished, elderly, and patients who are obese; those with impaired immune systems and chronic illness (eg, diabetes); and those on long-term corticosteroid therapy or immunosuppressive agents. The condition may be prevented by prompt treatment and management of focal and soft tissue infections. Clinical Manifestations · When the infection is bloodborne, onset is sudden, occurring with clinical manifestations of sepsis (eg, chills, high fever, rapid pulse, and general malaise). Assessment and Diagnostic Findings · Acute osteomyelitis: Early x-ray films show only soft tissue swelling.
These labels are meant to denote a greater hazard than that posed by any biological product gastritis diet beverages discount renagel 400 mg online. Using biohazard labels on all products without rationale that is documented in facility records is considered a deficiency gastritis en ninos purchase renagel 800 mg with mastercard. Since autologous recipients are not at risk of contracting a communicable disease from themselves (they already have the disease) gastritis diet ������ renagel 400mg amex, the statement "Warning: Advise patient of communicable disease risk" is not required on autologous product labels even if donor testing results are positive gastritis head symptoms purchase 800 mg renagel mastercard, although a biohazard label is required. Once regulated products have reached the stage of licensure, the label or accompanying records must include the statement "Rx Only" indicating that the product may only be distributed by a prescription from the transplant physician. Examples of all labels in use by the applicant facility will be provided to the inspector prior to the on-site inspection. For applicant programs performing both allogeneic and autologous cellular therapy, examples of labels will include collection, processing, transport, and distribution labels for both types. Tie tags, instructions to the infusionist, biohazard labels, and warning labels should also be included. This will maximize the efficiency of the inspection by allowing the inspector to focus on elements that can only be verified on-site. The inspector should further verify that labels are available for every type of cellular therapy product collected, with suitable modifications. Autologous product labels should be examined to confirm that "Not Evaluated for Infectious Substances" is present when the donor screening and testing does not contain all of the elements listed in B6. Such products must contain this statement, attached or affixed to the label or accompanying the product. For example, this statement must be on the following: A product not tested at all for relevant communicable disease agents and diseases. A product tested for only a subset of relevant communicable disease agents and diseases. A product screened and tested for all relevant communicable disease agents and diseases but using diagnostic tests rather than donor screening tests. A product screened and tested for all relevant communicable disease agents and diseases using approved donor screening test, but for which no official donor eligibility determination was made. Any autologous product with the presence of risk factors for or clinical evidence of relevant communicable disease agents or diseases must have these two labels, whether or not the regulations for donor eligibility determination were completely followed. Labeling of the product before disconnecting it from the donor will prevent mix-up when there is more than one donor undergoing collection. If confidentiality is a concern, partial labels may be used until the product is disconnected from the donor. A statement is required attesting to donor eligibility (or ineligibility) based on the screening and testing that was performed, a summary of the records used to make the donor eligibility determination, and the identity and address of the facility that made that determination. This summary must include results of the donor screening for infectious disease risk and the communicable disease test results. If the Collection Facility is responsible for allogeneic donor eligibility determination, that facility is also responsible for distributing the above information to the Clinical Program and Cell Processing Facility. If the Clinical Program determines allogeneic donor eligibility, the Collection Facility must obtain the information from the program so that it may accompany the product. Example(s): It is permissible to have hard copies of each item physically accompany the product, and in some cases, that may be most appropriate, as when a product leaves the Collection Facility and is transported to another institution for processing, storage, and/or administration. Explanation: If the Collection Facility participates in donor eligibility determination, completion of this determination must be documented. The type of anticoagulants and/or solutions added to the cell collection container during the procedure. Requirements for monitoring the donor prior to , during, and after collection (as applicable). If there is no collection procedure scheduled for the day of an onsite inspection, the inspector should ask the Collection Facility staff to perform a mock collection, including all parts of the donor interview and consent for which that facility is responsible, and all labeling and storage steps. Questions may be asked to determine: Are cellular therapy products from different donors stored in the Collection Facility at the same time? Is there a record of the lot numbers and expiration dates for all reagents used in collection?
Safe 400mg renagel. What is Okra Good For? 5 Wonderful Benefits of Okra.
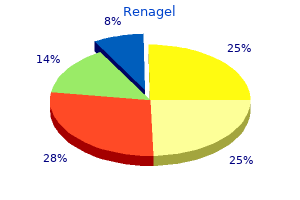
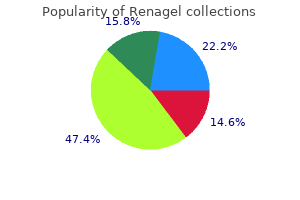
Bengal Quince (Bael). Renagel.
- Dosing considerations for Bael.
- What is Bael?
- Are there safety concerns?
- How does Bael work?
- Constipation and diarrhea.
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96201
If we should fail to succeed in our efforts at a digital transformation of our company gastritis diet ������ renagel 400mg line, then there is a risk that we may fail to create the innovative new products gastritis diet advice nhs discount renagel 400mg mastercard, tools or techniques that such technologies may make possi- ble gastritis korean purchase renagel 800mg without a prescription, or may fail to create them as quickly and efficiently as such technologies may enable gastritis healing diet generic 400 mg renagel amex. We may also lose opportunities to engage with our stakeholders and to profit from improved business processes, and may lose the resources devoted to these efforts to transform our business. At the same time, should third parties successfully enter the healthcare field with disruptive new technologies or business models, then we potentially may see our business supplanted in whole or in part by these new entrants. To provide additional information that may be useful to investors, including changes in sales volume, we present information about our net sales and various values relating to operating and net income that are adjusted for such foreign currency effects. However, in performing our evaluation, we also consider equivalent measures of performance that are not affected by changes in the relative value of currencies. Free cash flow is a measure of the net cash generated that is available for debt repayment, investment in strategic opportunities and for returning to shareholders. Novartis defines free cash flow as cash flow from operating activities and cash flow associated with the purchase or sale of property, plant and equipment, as well as intangible, other non-current and financial assets, excluding marketable securities. The definition of free cash flow used by Novartis does not include amounts related to changes in investments in associated companies or related acquisitions or divestments of subsidiaries. Novartis defines net debt as current and non-current financial debt less cash and cash equivalents, current investments and derivative financial instruments. Impairments: Cost of goods sold and Research & Development include impairment charges related to intangible assets; Research & Development and Other expense include impairment charges related to financial assets; Research & Development, Other income and Other expense include reversals and charges related to the impairment of property, plant and equipment. Acquisition or divestment of businesses and related items, including restructuring and integration charges: Other income and Other expense include transitional service-fee income and expenses and other items related to the portfolio transformation. Generally, this results in amortization and impairment of intangible assets and acquisition-related restructuring and integration items having a full tax impact. Impairments: Cost of goods sold and Research & Development include impairment charges related to intangible assets; Other income includes impairment reversals of property, plant and equipment; Other expense includes impairment charges related to property, plant and equipment, and financial assets. Acquisition or divestment of businesses and related items, including restructuring and integration charges: Other income and Other expense include transitional service-fee income and expenses and other items related to the portfolio transformation; Other income also includes a gain from the revaluation of a previously held financial investment in a newly acquired company. There is usually a tax impact on other items, although this is not always the case for items arising from legal settlements in certain jurisdictions. Adjustments related to income from associated companies are recorded net of any related tax effect. Significant accounting policies the Novartis Group (Novartis or Group) is a multinational group of companies specializing in the research, development, manufacturing and marketing of a broad range of healthcare products led by innovative pharmaceuticals and also including eye care products and cost-saving generic pharmaceuticals. They are prepared in accordance with the historical cost convention except for items that are required to be accounted for at fair value. The preparation of financial statements requires management to make certain estimates and assumptions, either at the balance sheet date or during the year that affect the reported amounts of assets and liabilities, including any contingent amounts, as well as of revenues and expenses. The functional currency of subsidiaries is generally the local currency of the respective entity. This reflects the fact that the cash flows and transactions of these entities are primarily denominated in these currencies. In cases where Novartis does not fully own a subsidiary, it has elected to value any remaining outstanding non-controlling interest at the time of acquiring control of the subsidiary at its proportionate share of the fair value of the net identified assets. Investments in associated companies (generally defined as investments in entities in which Novartis holds between 20% and 50% of voting shares or over which it otherwise has significant influence) and joint ventures are accounted for using the equity method, except for selected venture fund investments for which the Group has elected to apply the method of fair value through the consolidated income statement. Acquisition of assets Acquired assets are initially recognized on the balance sheet at cost if they meet the criteria for capitalization. If acquired as part of a business combination, the fair value of identified assets represents the cost for these assets. If separately acquired, the cost of the asset includes the purchase price and any directly attributable costs for bringing the asset into the condition to operate as intended. Expected costs for obligations to dismantle and remove property, plant and equipment when it is no longer used are included in their cost. Property, plant and equipment Property, plant and equipment are depreciated on a straight-line basis in the consolidated income statement over their estimated useful lives. Leasehold land is depreciated over the period of its lease, whereas freehold land is not depreciated. The related depreciation expense is included in the costs of the functions using the asset. The following table shows the respective useful lives for property, plant and equipment: Useful life Buildings Machinery and other equipment Machinery and equipment Furniture and vehicles Computer hardware 20 to 40 years 7 to 20 years 5 to 10 years 3 to 7 years tial impairment whenever facts and circumstances indicate that their carrying value may not be recoverable.
Once a patient has been enrolled chronic gastritis flatulence discount renagel 800mg free shipping, they will be contacted by Amgen Safety Net Foundation to provide consent for shipment gastritis diet juice purchase renagel 800 mg otc. Amgen Reimbursement Counselors can assist with submitting gastritis diet ���� cheap 400 mg renagel amex, storing gastritis burping buy generic renagel 800mg online, and retrieving benefit verifications for anyone currently on an Amgen product. The Benefit Verification Center offers the tools, information, and support that make a difference to providers and patients. Independent Nonprofit Programs For patients with government insurance (like Medicare), Amgen Assist 360 can refer patients to independent nonprofit patient assistance programs that may be able to help them afford the co-pay costs for their prescribed medicine. Amgen has no control over these programs and provides referrals as a courtesy only. Uninsured Patients Patients may be able to receive Amgen medications at no cost from Amgen Safety Net Foundation (amgensafetynetfoundation. To enroll Padcev, Xospata, or Xtandi Support Solutions, visit astellaspharmasupportsolutions. To enroll, fill out the appropriate section during the Xospata Support Solutions enrollment process. To enroll a patient in the Astellas Patient Assistance Program, 16 Xospata Support Solutions (astellas pharmasupportsolutions. It provides information regarding patient healthcare coverage options and financial assistance information that may be available to help patients with financial needs. The Xospata Copay Card Program is for eligible patients who have commercial prescription insurance. The Program parameters are as follows: · Patients pay as little as $0 per prescription · A patient will be enrolled in the program for a 12-month period · the program benefit covers up to a maximum of $25,000 per calendar year · There are no income requirements. Xospata Support Solutions can evaluate eligibility and enroll patients in the Xospata co-pay card program, or the preferred network specialty pharmacy can be contacted to determine eligibility and enroll the patient in the program. The program is not valid for patients whose prescription claims are reimbursed, in whole or in part, by any state or federal government Xospata Quick Start+? Program the Xospata Quick Start+ Program provides a one-time, 7-day supply of Xospata at no cost to eligible patients who experience an insurance-related delay. Patients can receive their savings card by contacting their specialty pharmacy or by applying at activatethecard. The program is not valid for patients whose prescription claims are reimbursed, in whole or in part, by any state or federal government program, including, but not limited to , Medicaid, Medicare, Medigap, Department of Defense, Veterans Affairs, TriCare, Puerto Rico government insurance, or any state patient or pharmaceutical assistance program. It provides information regarding patient healthcare coverage options and financial assistance options that may be available to help patients with financial needs. Under this Program: · Patients pay as little as $5 per dose · A patient will be enrolled in the Program for a 12-month period · Patients may save up to a maximum of $25,000 per calendar year · There are no income requirements. Padcev Support Solutions can evaluate eligibility and enroll patients in the Padcev Copay Assistance Program, or patients can enroll through the Padcev Patient Enrollment Process. Xtandi Quick Start+? Program the Xtandi Quick Start+ Program provides a one-time, 14-day supply of Xtandi at no cost to new patients who experience a delay in insurance coverage. To enroll, fill out the appropriate section during the Xtandi Support Solutions enrollment process. It provides information regarding patient healthcare coverage, financial assistance information that may be available to help patients with financial needs, and coding and billing information for Padcev. The Xtandi Patient Savings Program is for eligible patients who have commercial prescription insurance. If the patient is eligible for the program, Padcev Support Solutions will notify the provider and the patient. Astellas Pharma Support Solutions will follow up with the insurer to confirm receipt, check status, and obtain the outcome. Patients may pay $0 per supply or infusion, dependant on the specific medication, and subject to annual maximums. If the patient meets the eligibility requirements, providers can enroll patients into the Patient Savings Program via the online enrollment portal. The links to the portal for each product can be found at astrazenecaspecialtysavings. Once enrolled, patient-specific account information will be presented in the portal for immediate use.